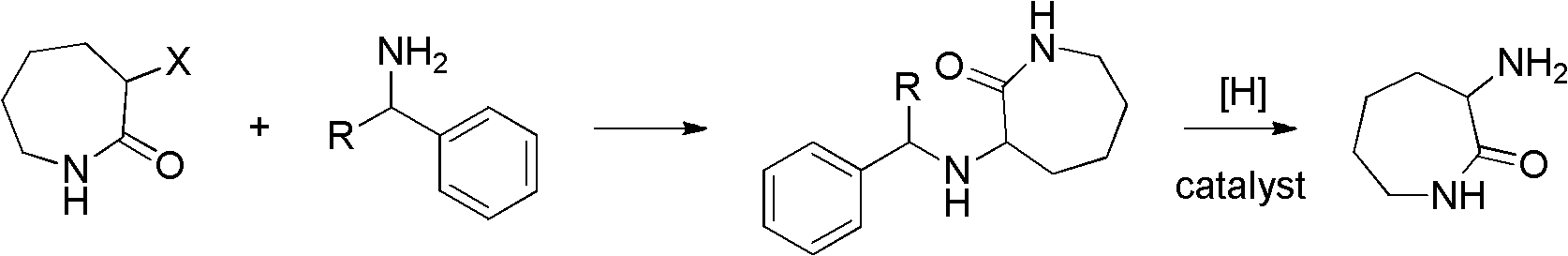
Method for synthesizing DL-alpha-amino caprolactam
An aminocaprolactam, caprolactam technology, applied in the direction of organic chemistry and the like, can solve the problems of complicated purification, complicated operation, many reaction steps, etc., and achieves the effects of safe operation, easy availability of raw materials, and easy separation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
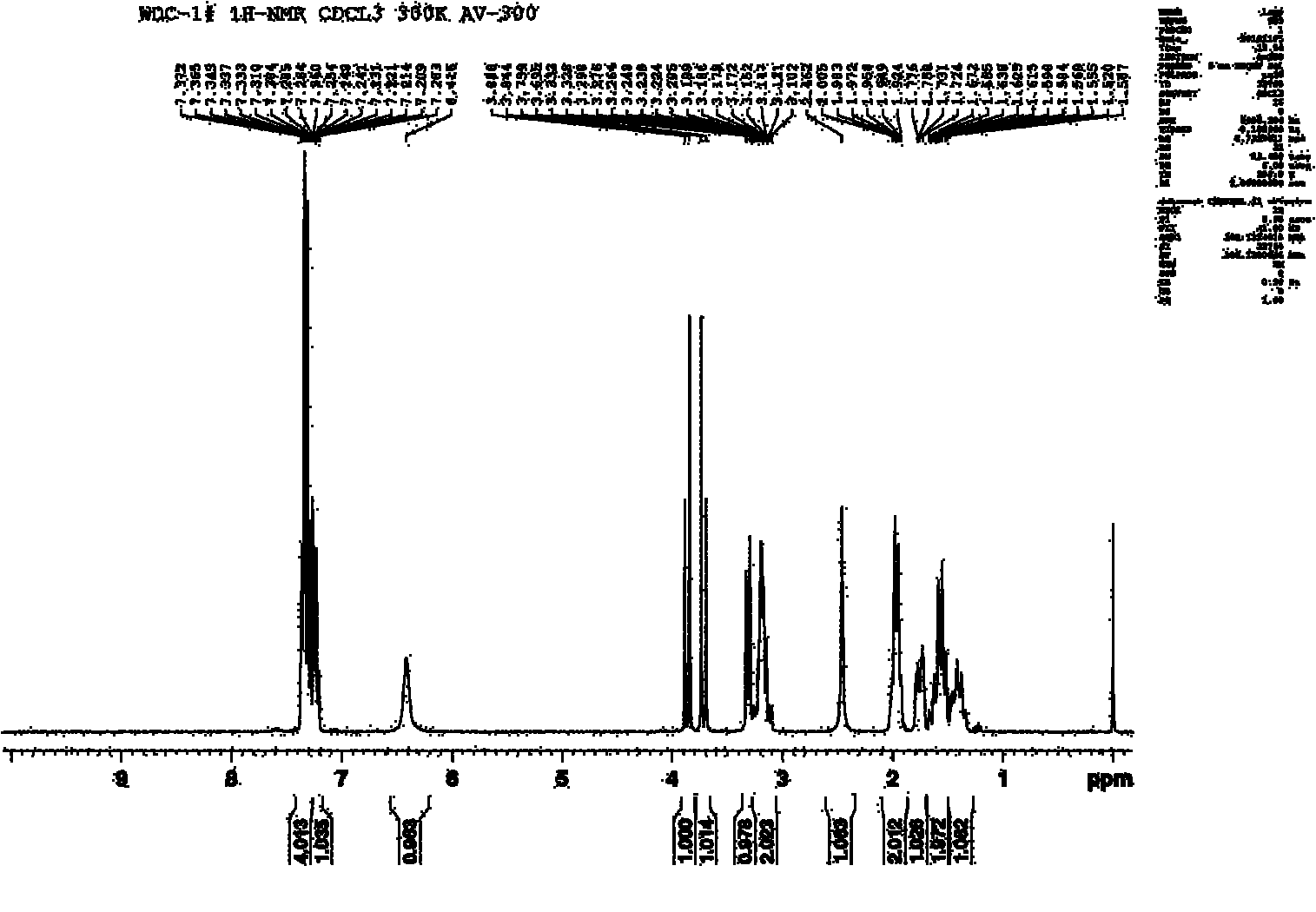
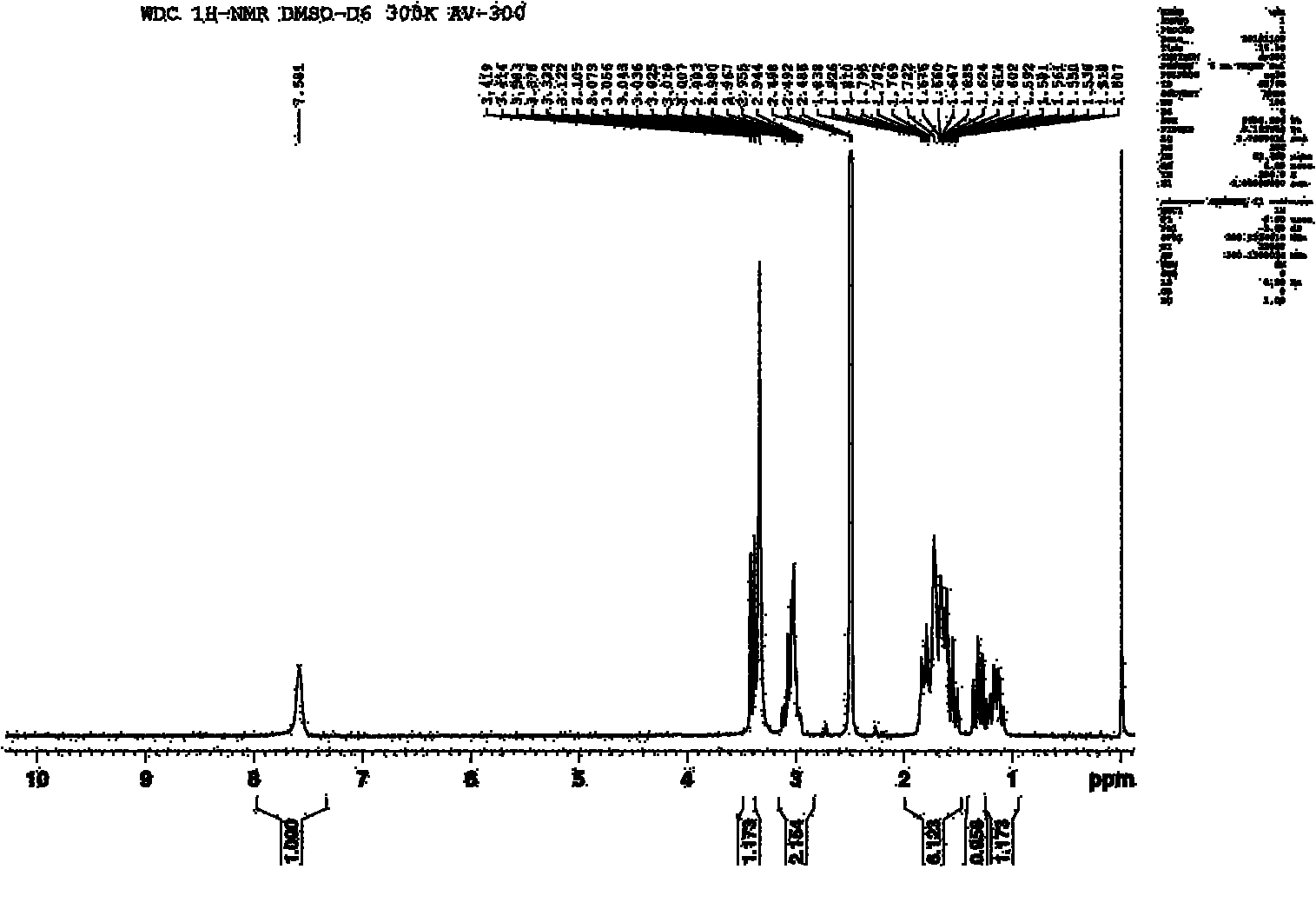
[0022] α-Chlorocaprolactam (14.8g, 0.1mol) and benzylamine (11.8g, 0.11mol), K 2 CO 3 (13.7g, 0.1mol), N,N-dimethylformamide (50mL) were added to the reactor together, the temperature was raised to 145°C, and after constant temperature reaction for 4h, it was poured into 200ml of water, and the solid was precipitated by stirring, filtered, washed with water, After drying, 18.1 g of N-benzyl-DL-α-aminocaprolactam was obtained, with a yield of 81.04%. The obtained N-benzyl-DL-α-aminocaprolactam proton nuclear magnetic resonance spectrum is as follows figure 1 shown. Add 18.1g of N-benzyl-DL-α-aminocaprolactam into the reactor, add 1g of 10% Pd-C and 3.82g of formic acid, reflux in 50ml of methanol for 4h, stop the reaction, filter, spin dry the solvent, ethyl acetate The ester was recrystallized to obtain 7.1 g of the target product DL-α-aminocaprolactam, with a yield of 66.81%. The obtained DL-α-aminocaprolactam nuclear magnetic resonance spectrum is as follows figure 2 s...
Embodiment 2
[0024] α-Chlorocaprolactam (14.8g, 0.1mol) and benzylamine (16.1g, 0.15mol), K 2 CO 3 (13.7g, 0.1mol), N,N-dimethylformamide (50mL) were added to the reactor together, the temperature was raised to 145°C, and the constant temperature reaction was carried out for 4h, poured into 200ml of water, stirred to precipitate a solid, filtered, washed with water, and dried 18.5 g of N-benzyl-DL-α-aminocaprolactam was obtained, with a yield of 84.75%. Add 18.5g of N-benzyl-DL-α-aminocaprolactam into the reactor, add 2g of 5% Pd-C and 5g of formic acid, reflux in 50ml of methanol for 3.5h, stop the reaction, filter, spin dry the solvent, ethyl acetate The ester was recrystallized to obtain 7.3 g of the target product DL-α-aminocaprolactam, with a yield of 67.2%. [Mp: 68.2-71℃; FAB-MS m / z: 129.1[M+1] + ]
Embodiment 3
[0026] α-Chlorocaprolactam (44.3g, 0.3mol) and benzylamine (112.5g, 1.05mol), K 2 CO 3 (27.4g, 0.2mol), 1,2-propanediol (150mL) were added to the reactor together, the temperature was raised to 100°C, the temperature was reacted for 3h, cooled to room temperature, added to 500ml of water, stirred to precipitate a solid, filtered, washed with water, and dried 50.6 g of N-benzyl-DL-α-aminocaprolactam was obtained, with a yield of 77.23%. Add 50.6 g of the obtained N-benzyl-DL-α-aminocaprolactam into the reactor, add 5 g of 10% Pd-C and 10.7 g of formic acid, reflux in 150 ml of ethanol for 3 hours, stop the reaction, filter, spin dry the solvent, acetic acid Ethyl ester was recrystallized to obtain 19.5 g of the target product DL-α-aminocaprolactam, with a yield of 65.63%. [Mp: 68.2-70.6℃; FAB-MS m / z: 129.1[M+1] + ]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com