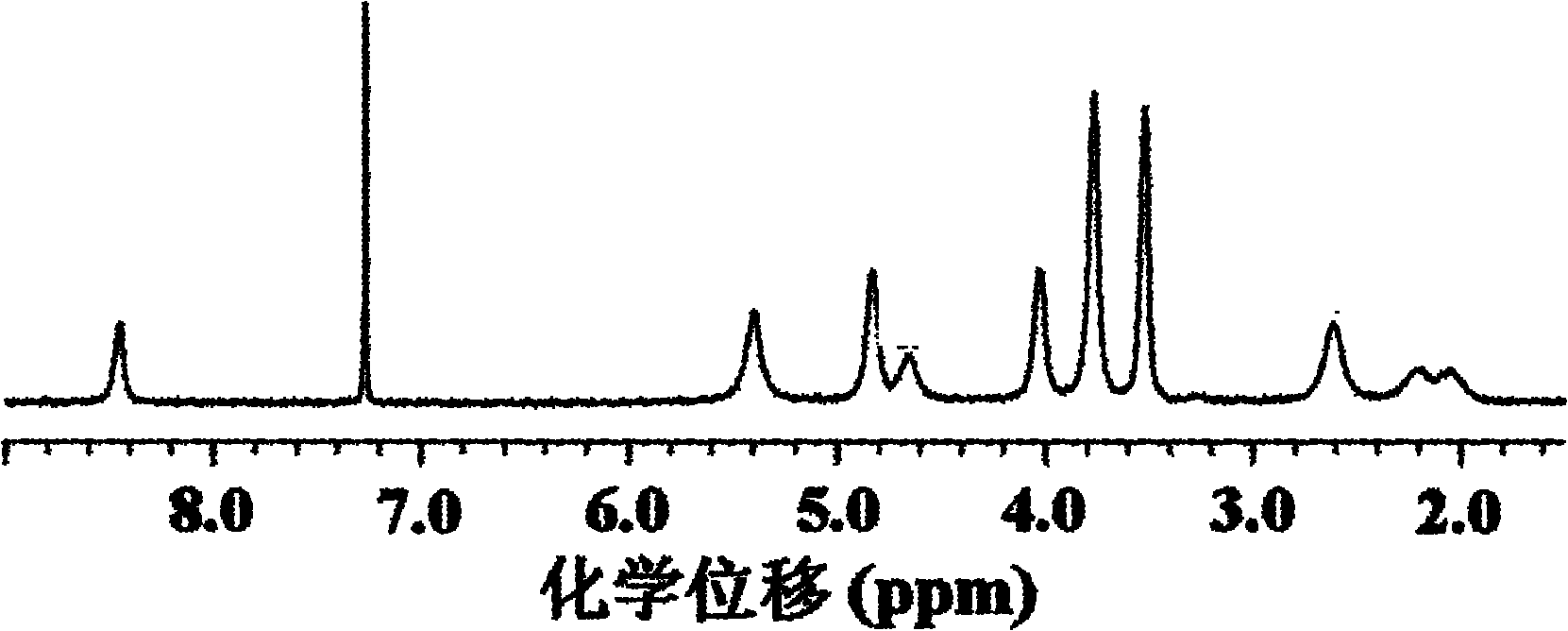
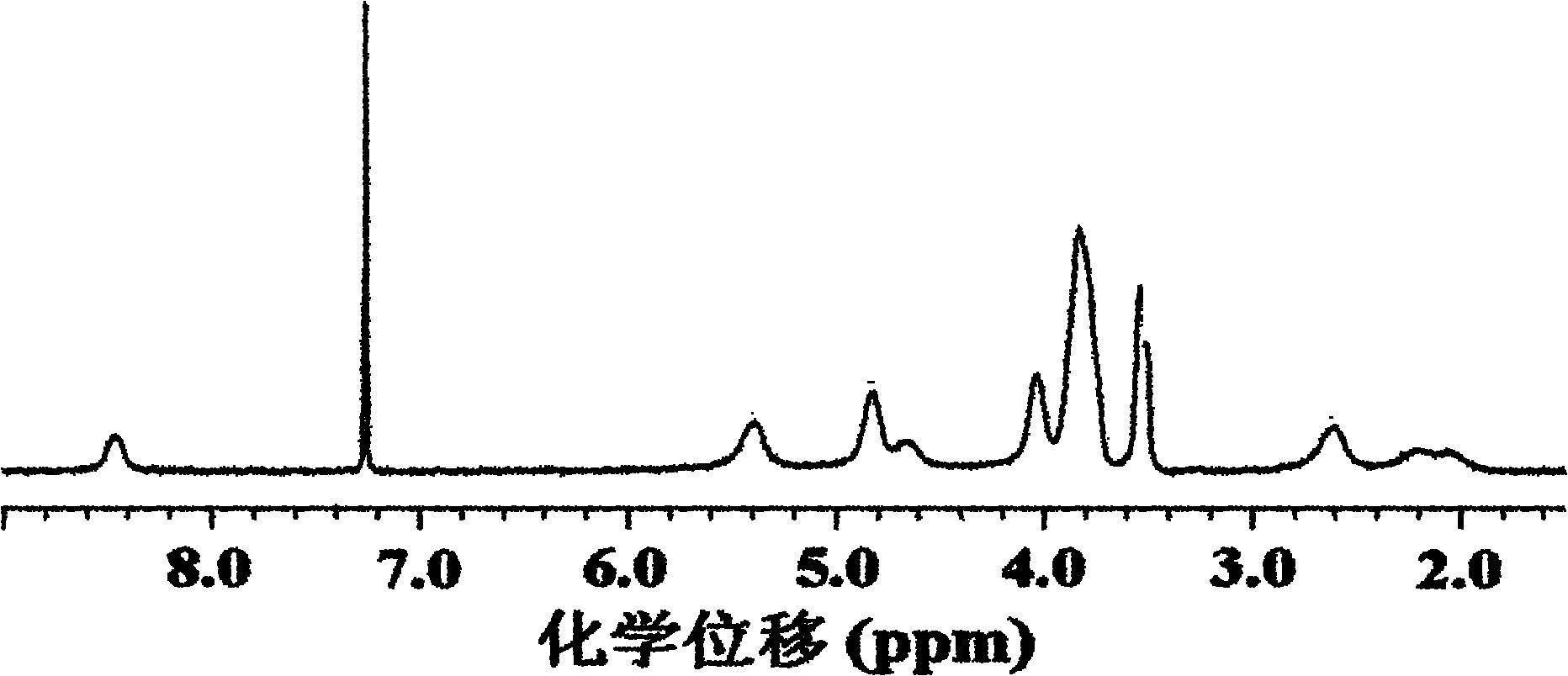
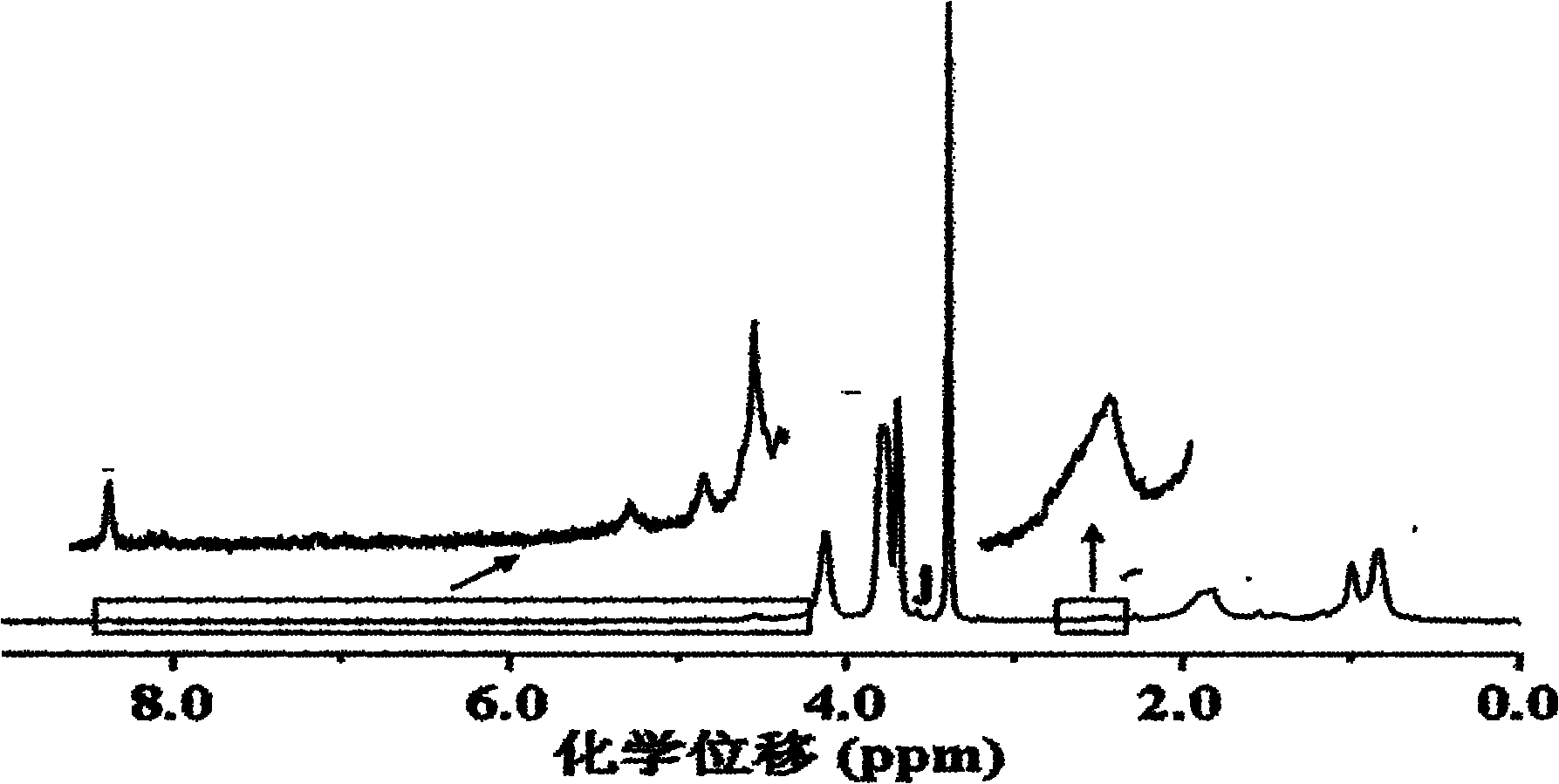
Poly(L-glutamic acid) homopolymer, random copolymer and graft copolymer, and preparation methods thereof
A technology of random copolymer and graft copolymer is applied in the fields of poly(L-glutamic acid) homopolymer, random copolymer, graft copolymer and their preparation, and can solve the problems of small application range and the like, Achieve the effects of no toxic side effects, good biocompatibility and degradability, and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] The present invention also provides a kind of preparation method of poly(L-glutamic acid) homopolymer described in above-mentioned technical scheme, comprises the following steps:
[0044] Gamma-propynyl-L-glutamic acid ester-N-internal carboxylic acid anhydride is polymerized under the action of primary amine initiator to obtain poly(L-glutamic acid) homopolymer.
[0045] The present invention uses γ-propynyl-L-glutamic acid ester-N-internal carboxylic acid anhydride as raw material, uses primary amine initiator as initiator, and prepares poly(L-glutamic acid) homopolymer by atom transfer radical polymerization thing.
[0046] The γ-propynyl-L-glutamate-N-internal carboxylic acid anhydride is preferably prepared according to the following steps:
[0047] L-glutamic acid and propynyl alcohol react under the action of concentrated sulfuric acid to obtain γ-propynyl-L-glutamate;
[0048] The γ-propynyl-L-glutamate reacts with bis(trichloromethyl)carbonate at 40°C to 60°...
Embodiment 1
[0088] The preparation of embodiment 1 gamma-propynyl-L-glutamate
[0089] Mix 1mol L-glutamic acid and 3mol propynyl alcohol at 0°C, add 1.5mol concentrated sulfuric acid dropwise under the condition of stirring with a stirrer, after the dropwise addition, raise the temperature to 25°C for 24 hours, after the reaction, use 3mol Sodium bicarbonate solution neutralizes the reaction mixture, and after filtration, washing, recrystallization and freeze-drying, γ-propynyl-L-glutamic acid ester is obtained.
Embodiment 2
[0090] The preparation of embodiment 2 gamma-propynyl-L-glutamic acid ester-N-internal carboxylic acid anhydride
[0091] Mix 1 mol of γ-propynyl-L-glutamate prepared in Example 1 with 0.6 mol of bis(trichloromethyl)carbonate at 25°C, add tetrahydrofuran, heat to 50°C for 2 hours, and the reaction ends Finally, the reaction mixture was settled in excess petroleum ether, separated, washed, recrystallized and dried to obtain γ-propynyl-L-glutamic acid ester-N-internal carboxylic acid anhydride.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com