Multiplex fluorescence PCR (Polymerase Chain Reaction) detection method and detection kit for typhoid/paratyphoid saimonella
A technology of Salmonella typhi and detection kit, which is applied in the direction of fluorescence/phosphorescence, biochemical equipment and methods, microbiological determination/inspection, etc., to achieve objective and accurate results and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] A multiple fluorescent PCR detection kit for typhoid and paratyphi Salmonella, said kit comprising:
[0043] Tailing primers for specifically amplifying the ViaB gene sequences of Salmonella typhi and Salmonella paratyphi C, the base sequences of which are shown in SEQ ID NO: 1 (ViaB-TF) and SEQ ID NO: 2 (ViaB-TR) ;
[0044]A molecular beacon probe for detecting the ViaB gene of Salmonella typhi and Salmonella paratyphi C, the base sequence of which is shown in SEQ ID NO: 3 (ViaB-P);
[0045] Tailing primers for specifically amplifying the fliC-a gene sequence of Salmonella paratyphi A, the base sequences of which are as SEQ ID NO: 4 (fliC-a-TF) and SEQ ID NO: 5 (fliC-a-TR) shown;
[0046] A molecular beacon probe for detecting the fliC-a gene of Salmonella paratyphi A, the base sequence of which is shown in SEQ ID NO: 6 (fliC-a-P);
[0047] A tailing primer for specifically amplifying the fliC-b gene sequence of Salmonella paratyphi beta, its base sequence is as SEQ...
Embodiment 3
[0091] Embodiment 3 carries out methodological evaluation to the kit described in embodiment 1
[0092] 1. Method
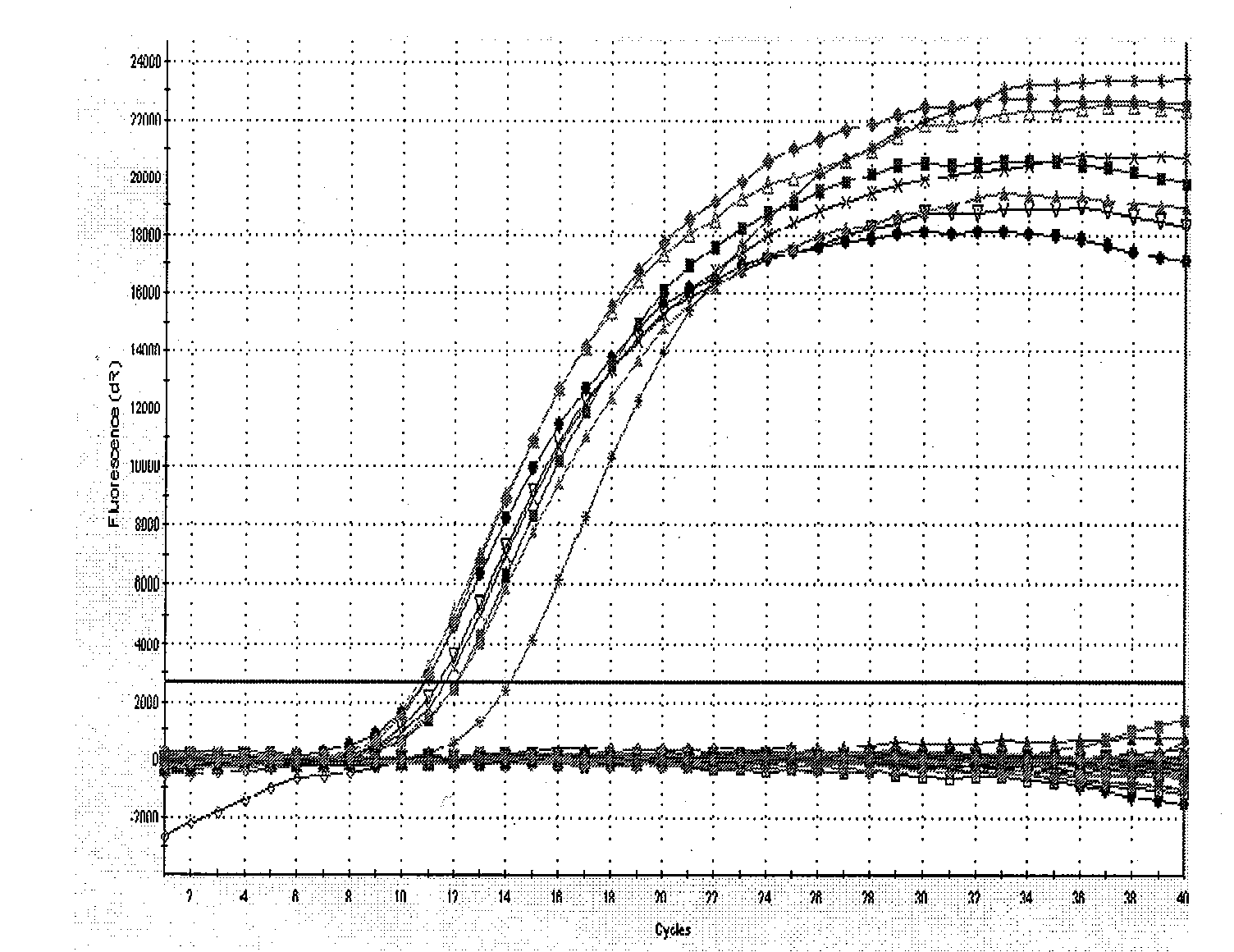
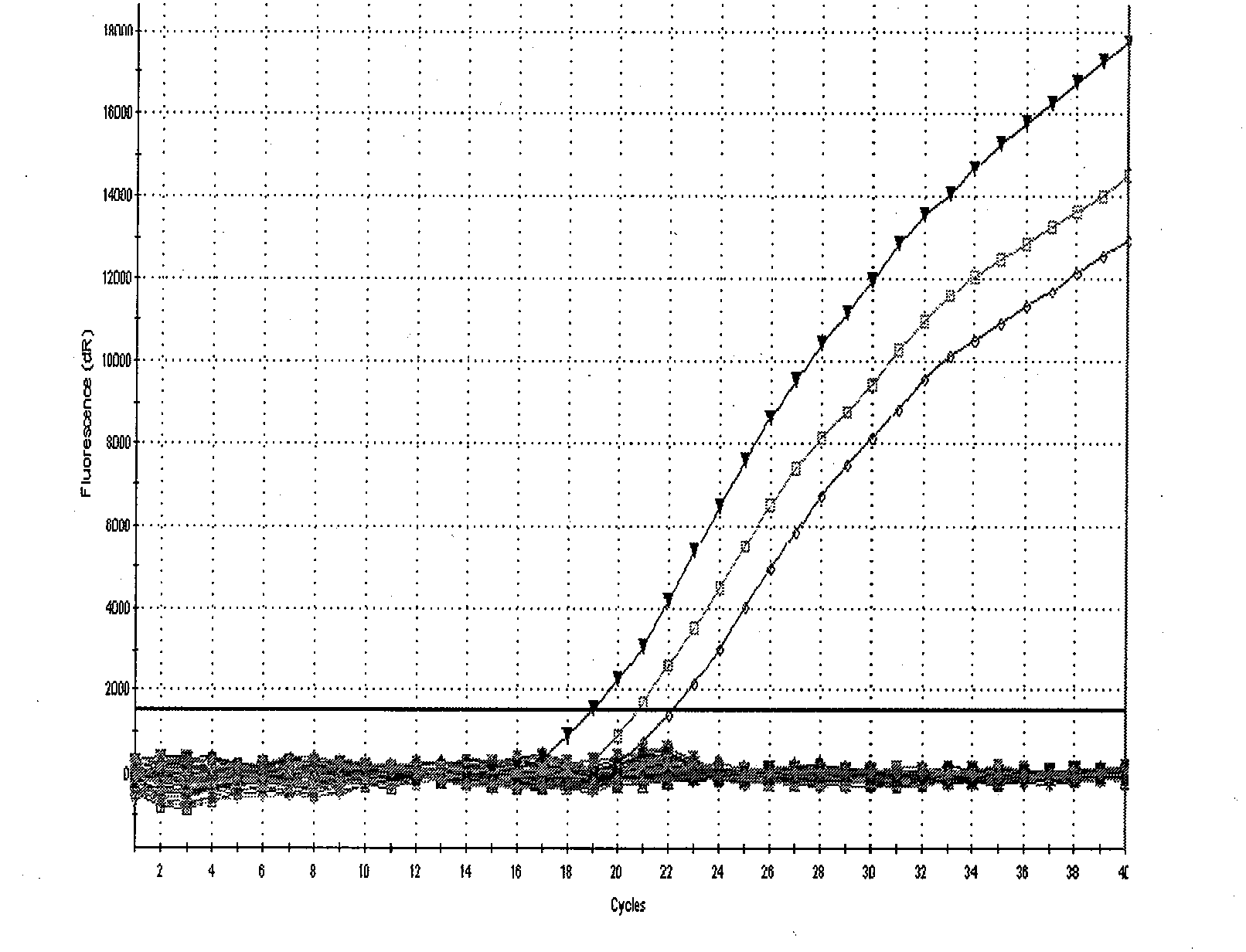
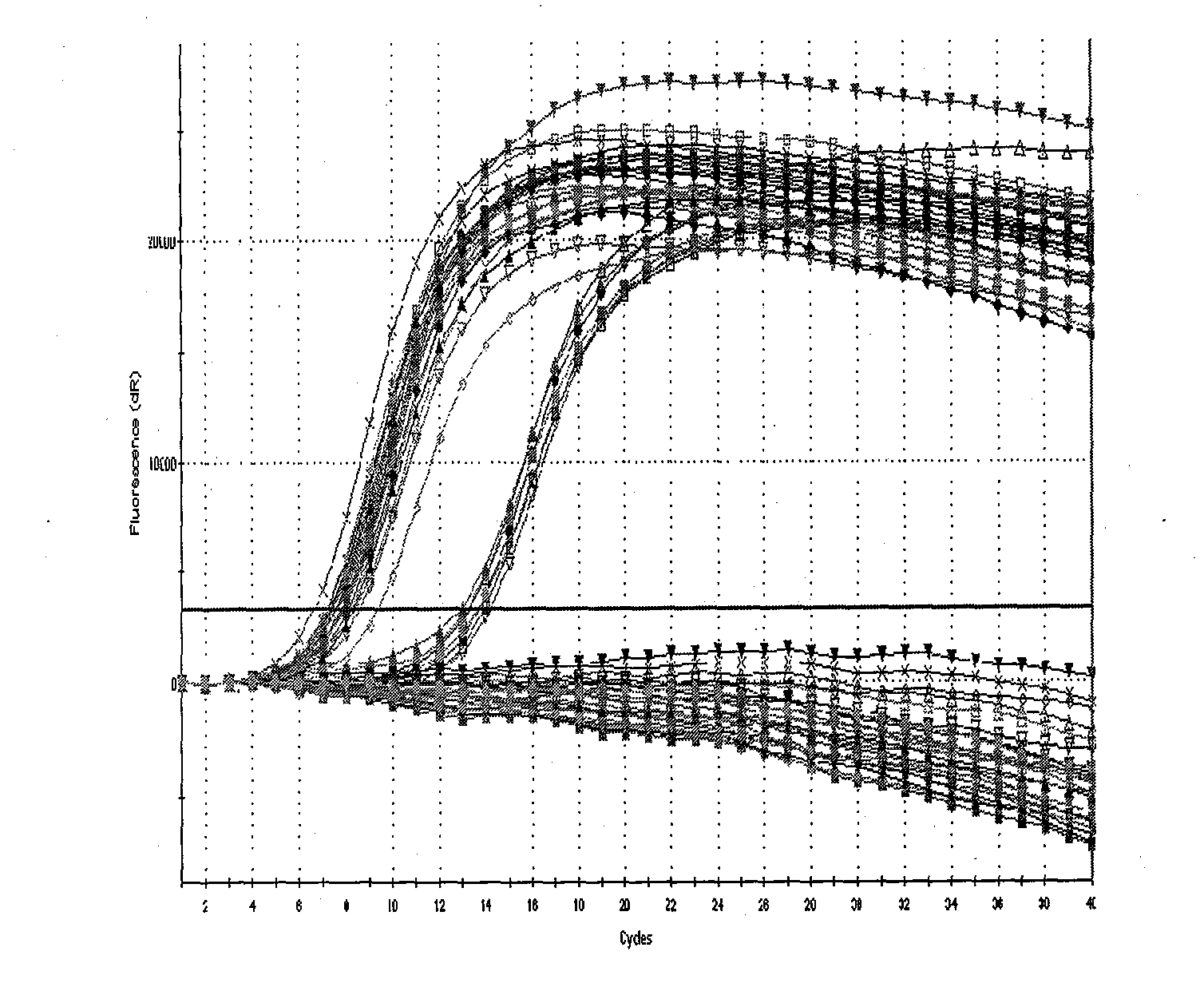
[0093] The 8 positive reference products and 8 negative reference products (see Table 4 for details) purchased from the Central Inspection Institute were serially diluted as required, and then the DNA was prepared according to the bacterial liquid boiling method (take 1ml bacterial liquid, centrifuge at 12000rpm for 5min, discard Supernatant: Add 50 μl of double distilled water to suspend the precipitate, boil at 100°C for 5 minutes, centrifuge at 12000 rpm for 2 minutes to take the supernatant as a template), and carry out experiments on the specificity, sensitivity, precision and detection limit of the fluorescent PCR system. Among them, the precision experiment needs to process 10 samples of the precision reference product in the same batch, and calculate the Ct value CV% in the test.
[0094] For the recipe and PCR program of the PCR system used, see Multipl...
Embodiment 4 Embodiment 1
[0099] Embodiment 4 Embodiment 1 described kit clinical assessment comparative experiment
[0100] Use the typhoid and paratyphi Salmonella multiplex PCR detection kit and gold standard culture method described in this application to carry out blind detection on blood samples. The negative and positive test results of the specimen are based on the culture method, and the detection performance indicators such as sensitivity and specificity of the present invention are measured with this.
[0101]A total of 538 cases of clinical trial samples from 3 provincial laboratories were detected, 212 cases were detected positive and 326 cases were negative by culture method; 218 cases were detected by the kit of the present invention and 320 cases were negative. Compared with the culture method, the sensitivity of the kit of the present invention to detect Salmonella typhi and paratyphi is 100%, and the specificity is 98.2%, as shown in Table 5.
[0102] Table 5 culture method compares ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com