Method for simultaneously detecting main component contents of quinapril hydrochloride and hydrochlorothiazide composition
A technology of quinapril hydrochloride and hydrochlorothiazide, which is applied in the direction of measuring devices, material separation, and analysis of materials, can solve problems such as difficulties and large polarity differences, and achieve the effects of simple operation, high precision, and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
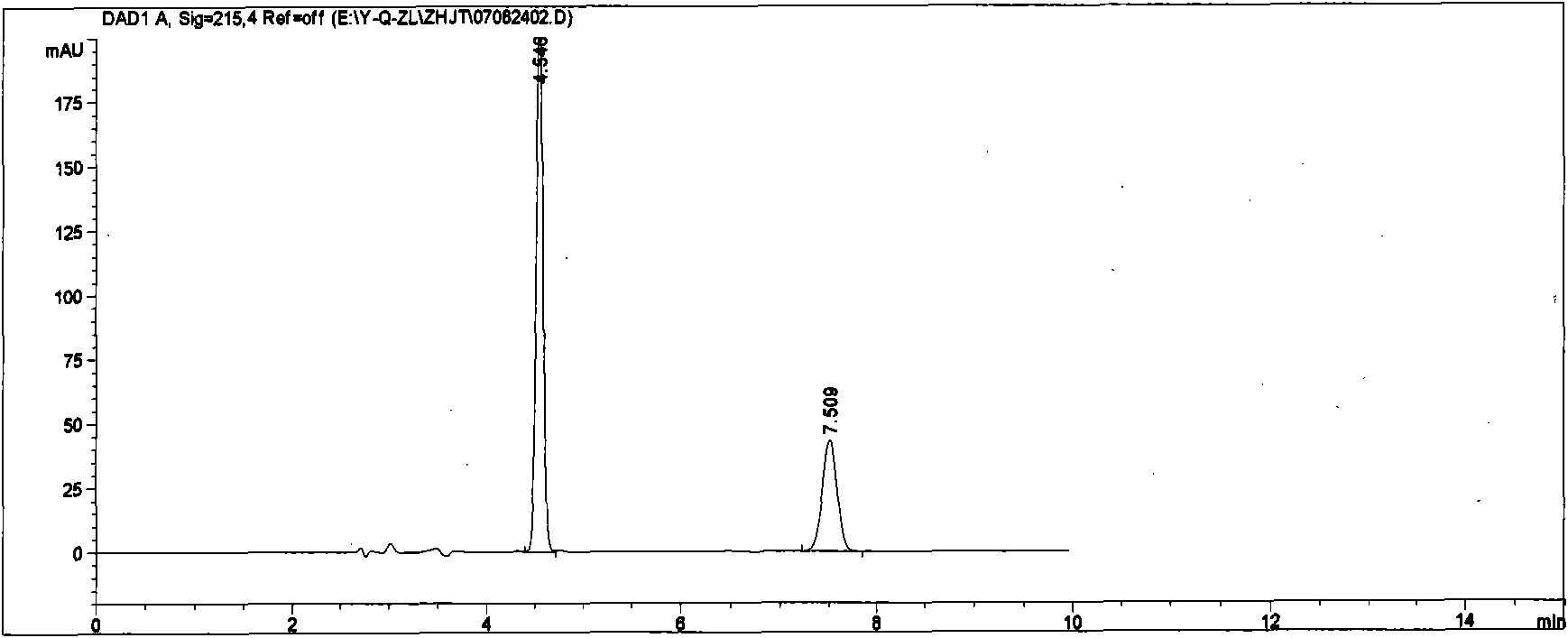
[0022] Chromatographic conditions: column Ultimate TM XB-CN (5μm, 4.6×250mm); column temperature: 40°C; mobile phase is acetonitrile: 0.05mol / L potassium dihydrogen phosphate solution (adjust pH 2.5 with phosphoric acid), 35:65; flow rate: 1ml / min ; Detection wavelength: 215nm; Injection volume: 20μl.
[0023] Under these chromatographic conditions, the peak eluting time of quinapril hydrochloride is 7.5 minutes, the peak eluting time of hydrochlorothiazide is 4.5 min, and the column efficiency is not less than 1000 based on the peak of quinapril hydrochloride.
[0024] Preparation of reference substance solution: Accurately weigh quinapril hydrochloride reference substance (approximately equivalent to 10 mg of quinapril), and appropriate amount of hydrochlorothiazide reference substance after drying at 105°C for 1 hour (corresponding to the prescription amount of hydrochlorothiazide for 10 mg of quinapril) , dissolved in mobile phase, diluted to make a solution containing ab...
Embodiment 2
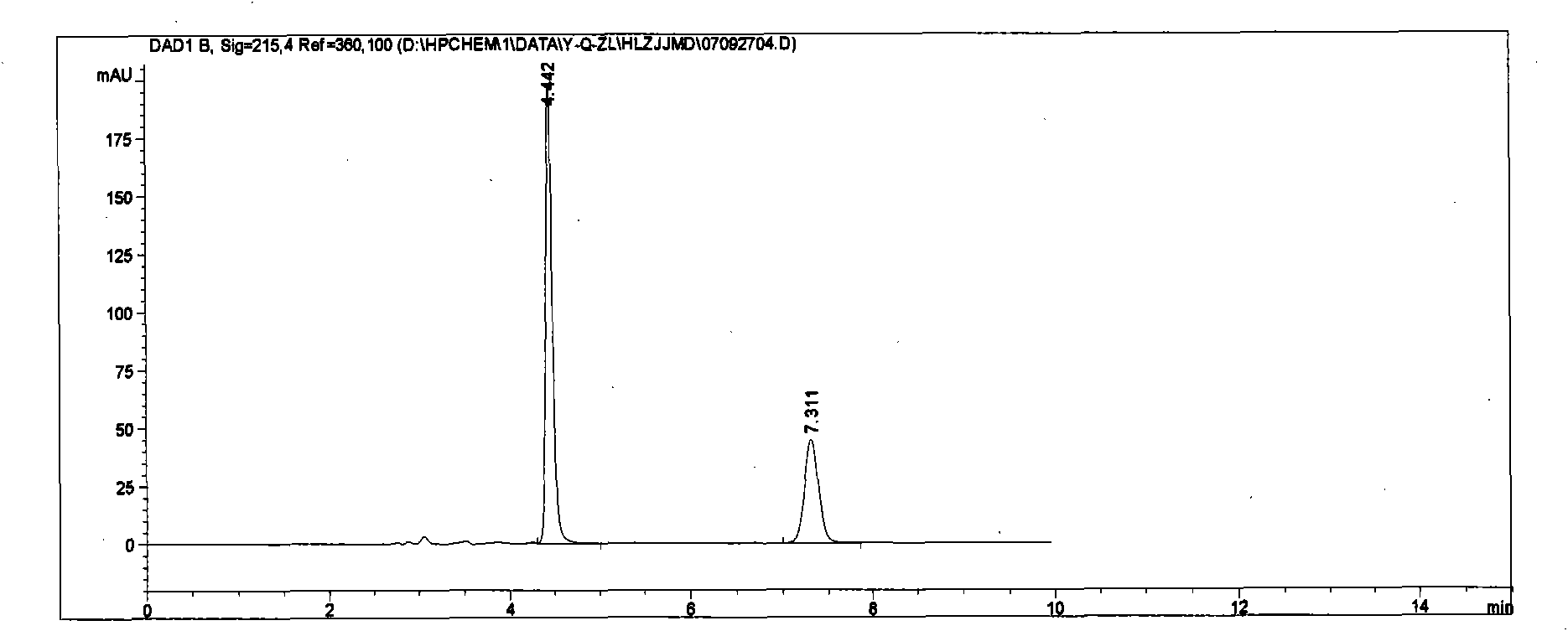
[0030] Chromatographic conditions: chromatographic column ZORBAX XDB-C8 (5μm, 4.6×250mm); column temperature: 30°C; mobile phase is acetonitrile: 0.05mol / L potassium dihydrogen phosphate solution (adjust pH 3.0 with phosphoric acid), 40:60 ; Flow rate: 1ml / min Detection wavelength: 215nm; Injection volume: 20μl.
[0031] Under these chromatographic conditions, the peak eluting time of quinapril hydrochloride is 7.0 min, the peak eluting time of hydrochlorothiazide is 4.0 min, and the column efficiency is better than 1000 in terms of quinapril hydrochloride peak.
[0032] Preparation of reference substance solution: Accurately weigh quinapril hydrochloride reference substance (approximately equivalent to 10 mg of quinapril), and appropriate amount of hydrochlorothiazide reference substance after drying at 105°C for 1 hour (corresponding to the prescription amount of hydrochlorothiazide for 10 mg of quinapril) , dissolved in mobile phase, diluted to make a solution containing ab...
Embodiment 3
[0037] Chromatographic conditions: column Ultimate TM XB-CN (5μm, 4.6×250mm); Column temperature: 40°C; Mobile phase is acetonitrile: 0.05mol / L potassium dihydrogen phosphate solution (adjust pH3.5 with phosphoric acid), 35:65; Flow rate: 1.2ml / min; detection wavelength: 215nm; injection volume: 20μl.
[0038] Under these chromatographic conditions, the peak elution time of quinapril hydrochloride is about 7.5 minutes, and the peak elution time of hydrochlorothiazide is about 4.5 minutes, and the column efficiency is not less than 1000 based on the peak of quinapril hydrochloride.
[0039] Preparation of reference substance solution: Accurately weigh quinapril hydrochloride reference substance (approximately equivalent to 10 mg of quinapril), and appropriate amount of hydrochlorothiazide reference substance after drying at 105°C for 1 hour (corresponding to the prescription amount of hydrochlorothiazide for 10 mg of quinapril) , dissolved in mobile phase, diluted to make a s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com