Triarylamine derivative with fluoro substituent and preparation method thereof
A fluorine substituent and derivative technology, which is applied in the field of triarylamine derivatives containing fluorine substituents and their preparation, can solve the problem of less types of triarylamine derivatives, and achieves good chemical stability, simple preparation method and convenient operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The reaction route is as follows:
[0029]
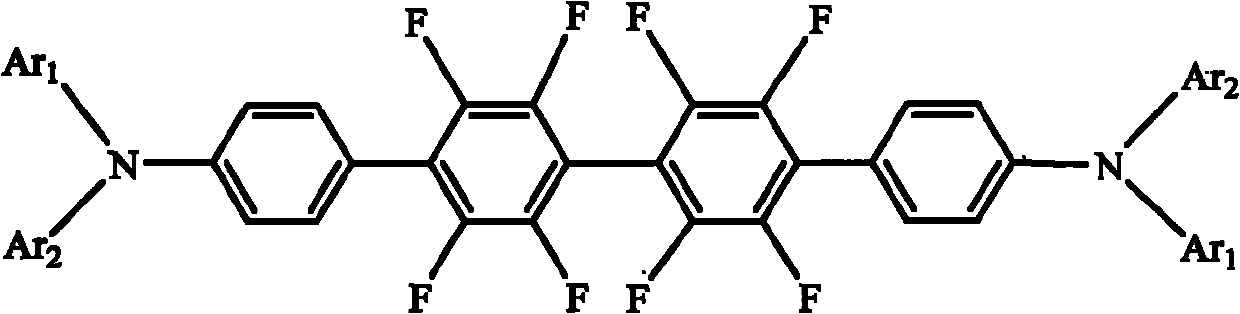
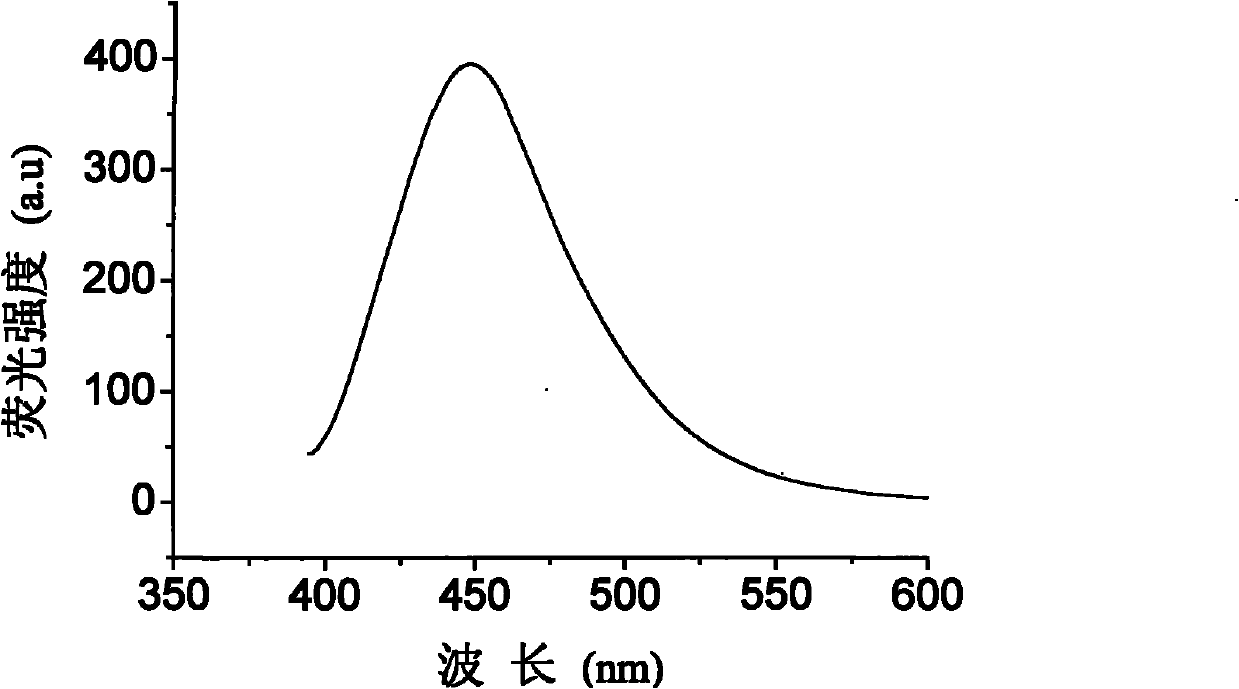
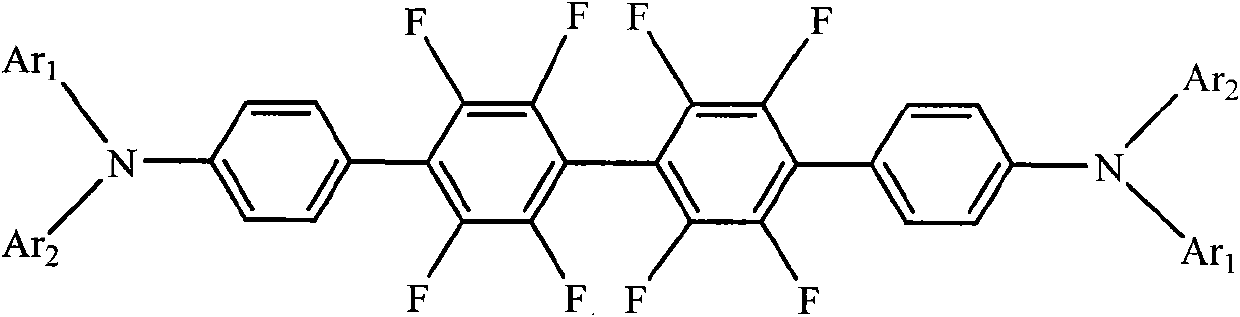
[0030] Take a clean 100mL flask, add 0.912g (2mmol) 4,4'-dibromooctafluorobiphenyl, 1.277g (4.4mmol) triphenylamine-4-boronic acid, 40mL toluene, 0.116g (0.1mmol) Pd into it (PPh 3 ) 4 , 6mL (12mmol) of K 2 CO 3 The aqueous solution, under nitrogen protection, was heated to 100 °C and stirred for 48 h. After cooling, the reaction mixture was washed with a large amount of distilled water until the water layer was clarified, the organic phase was separated with a separating funnel, and the organic phase was concentrated under reduced pressure. Finally, the concentrated crude product was subjected to a chromatography column (silica gel, petroleum ether, ethyl acetate) ester eluent) to obtain triarylamine derivatives containing fluorine substituents N,N,N',N'-tetraphenyl-[2',2",3',3",5',5",6 ',6"-octafluoro-p-tetraphenyl]-4,4"'-diamine, yield 67%. Solid photoluminescence (thin film, excitation wavelength 365nm): peak wave...
Embodiment 2
[0035] The reaction route is as follows:
[0036]
[0037] Others are the same as in Example 1, the triarylamine-based boronic acid used is N-phenyl-N-(3-methylphenyl)-4-aminophenylboronic acid, and the triarylamine derivative N,N'- Diphenyl-N,N'-bis(3-methylphenyl)-[2',2",3',3",5',5",6',6"-octafluoro-p-tetraphenyl ]-4,4"'-diamine, the others were similar to Example 1. The yield was 66%. Solid state photoluminescence (thin film, excitation wavelength 365 nm): peak wavelength 453 nm.
Embodiment 3
[0039] The reaction route is as follows:
[0040]
[0041]Others are the same as in Example 1, the triarylamine-based boronic acid used is N-phenyl-N-(1-naphthyl)-4-aminophenylboronic acid, and the triarylamine derivative N,N'-diphenyl containing fluorine substituents is synthesized base-N,N'-bis(1-naphthyl)-[2',2",3',3",5',5",6',6"-octafluoro-p-tetraphenyl]-4, 4"'-diamine, otherwise similar to Example 1. Yield 56%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com