Medicament of ilaprazole chemical structure and application thereof
A technology of ilaprazole and drugs, applied in the field of drugs with chemical structure of ilaprazole and its application, capable of solving problems such as increasing the risk of gastric cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Research on the Anti-Helicobacter Pylori Activity of Pharmaceutical Compositions Containing Ilaprazole
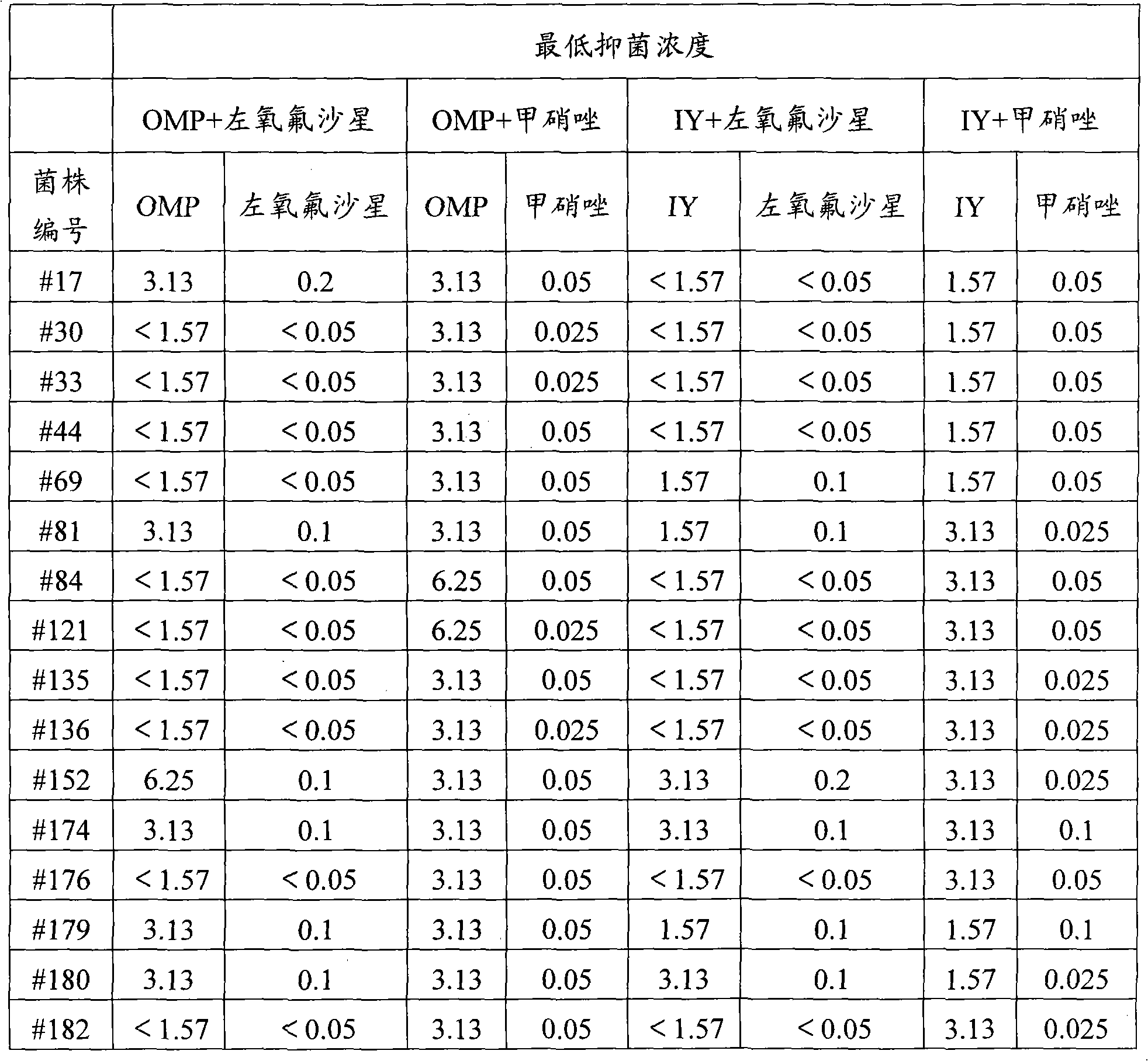
[0021] This example investigates the inhibitory effect of a pharmaceutical composition containing ilaprazole on clinical isolates of Helicobacter pylori. The minimum inhibitory concentrations (MICs) of dual drugs, that is, the pharmaceutical composition of ilaprazole and levofloxacin, and the pharmaceutical composition of ilaprazole and metronidazole on clinically isolated Helicobacter pylori strains were determined by agar dilution method .
[0022] Serially dilute ilaprazole and antibiotics (levofloxacin or metronidazole) 2 times with sterile water. Both azole (IY) and omeprazole (OMP) were 12.5-1.57 μg / ml. Table 1 shows the grouping situation of the dual drug combination and the minimum inhibitory concentration results against Helicobacter pylori.
[0023] Table 1 The minimum inhibitory concentration results (μg / ml) of anti-Helicobacter pylori of dua...
Embodiment 2
[0026] Example 2: Research on the Anti-Helicobacter Pylori Activity of Pharmaceutical Compositions Containing Ilaprazole
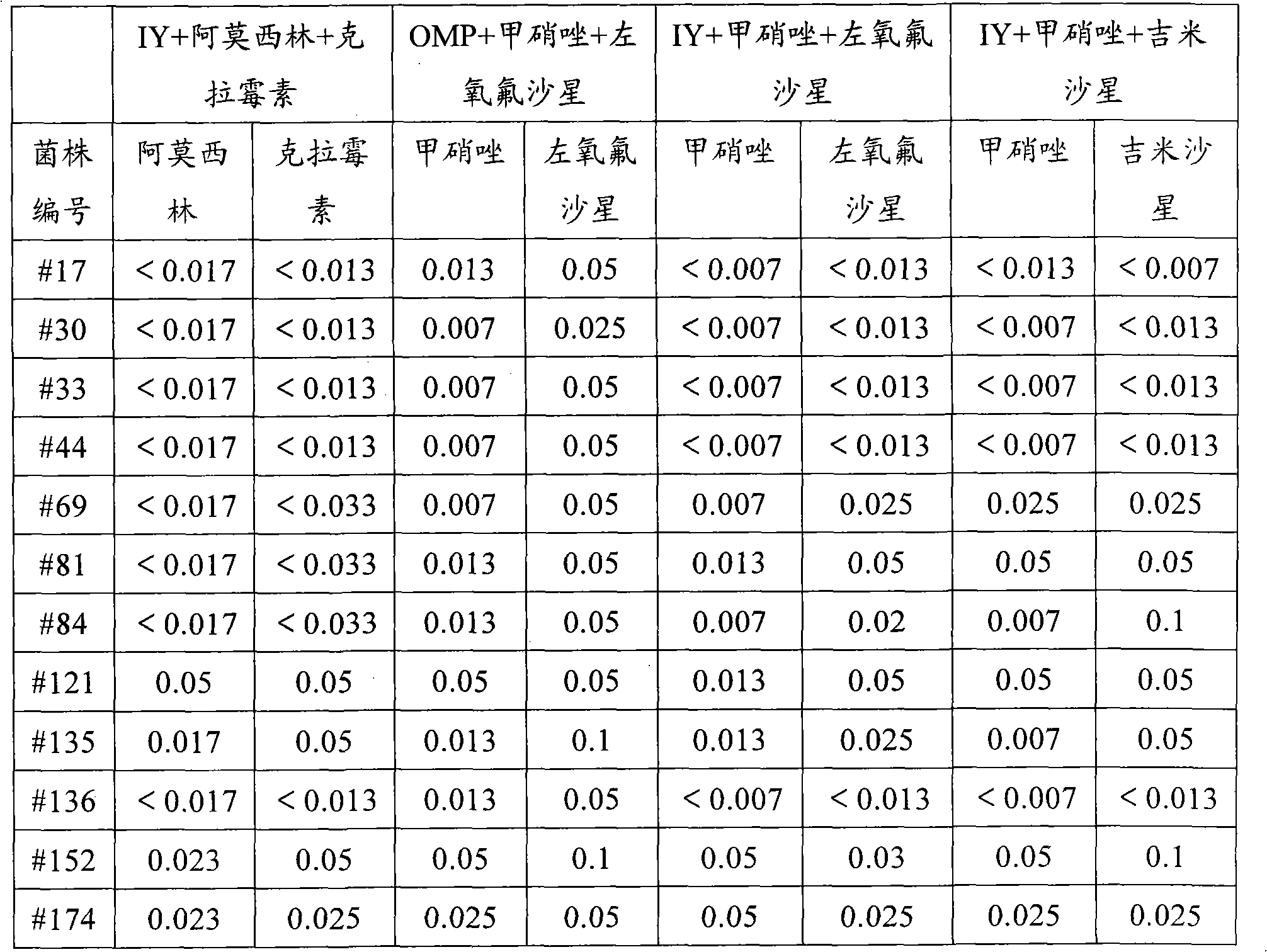
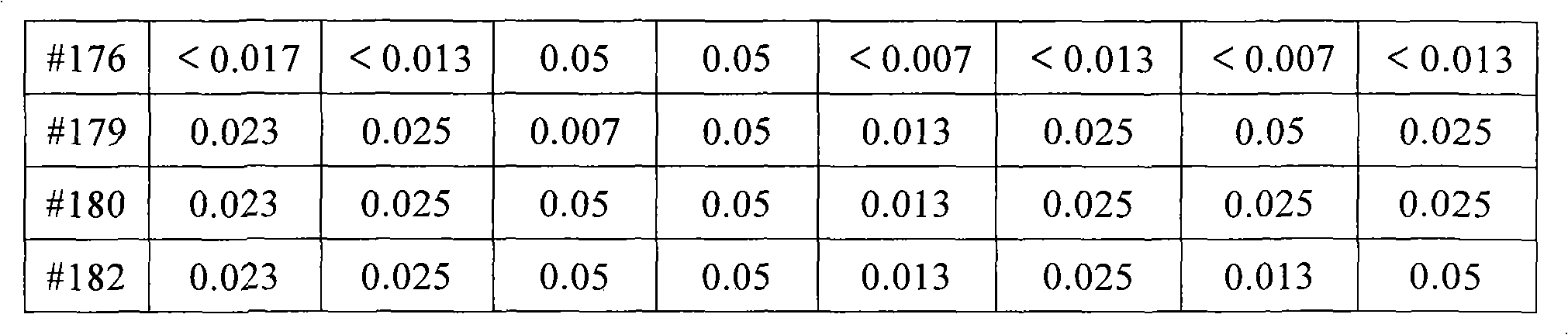
[0027] This example investigates the inhibitory effect of a pharmaceutical composition containing ilaprazole on clinical isolates of Helicobacter pylori. The minimum inhibitory concentrations (MICs) of the triple drug combination, i.e. ilaprazole, levofloxacin and metronidazole, against clinically isolated Helicobacter pylori strains were determined by agar dilution method.
[0028] Triple drugs are divided into the following 4 groups: ilaprazole (IY) 1.57μg / ml + metronidazole + levofloxacin; ilaprazole (IY) 1.57μg / ml + metronidazole + gemifloxacin; omeprazole (OMP ) 1.57 μg / ml+metronidazole+levofloxacin; ilaprazole (IY) 1.57 μg / ml+amoxicillin+clarithromycin triple drug anti-H. pylori minimum inhibitory concentration results are shown in Table 2.
[0029] Table 2 The minimum inhibitory concentration results (μg / ml) of triple drug anti-helicobacter pylori ...
Embodiment 3
[0033] Embodiment 3: the preparation method of pharmaceutical composition of the present invention
[0034] The preparation method of the pharmaceutical composition of the present embodiment comprises the following steps:
[0035] 1) Take materials according to the following parts by weight ratio: 1 part of ilaprazole, 100 parts of metronidazole, 200 parts of levofloxacin, 10-75 parts of microcrystalline cellulose, 5-50 parts of mannitol, hydroxypropylmethyl 0.2-30 parts of base cellulose, 1-30 parts of crospovidone, 0.1-5.0 parts of magnesium stearate; Azole and levofloxacin are passed through a 60-100 mesh nylon sieve, microcrystalline cellulose and crospovidone are respectively passed through a 60-100 mesh nylon mesh, and mannitol is crushed and passed through a 80-120 mesh (preferably 100 mesh) nylon mesh sieve ;
[0036] 3) The hydroxypropyl methylcellulose is formulated into an adhesive with a viscosity of 5-35 centipoise with 50-95% medicinal ethanol by volume percent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com