Method for preparing 2-(N-alkyl)aminobenzothiazole derivatives by using active alcohol as alkylating reagent
An alkylation reagent, the technology of aminobenzene, is applied in the field of preparation of 2-aminobenzothiazole derivatives to achieve the effect of high reaction atom economy and broad development prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
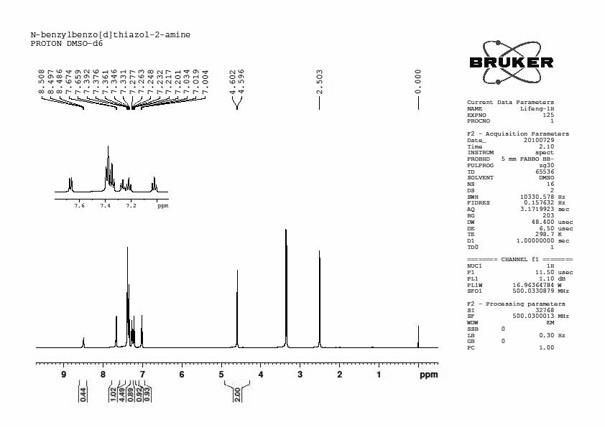
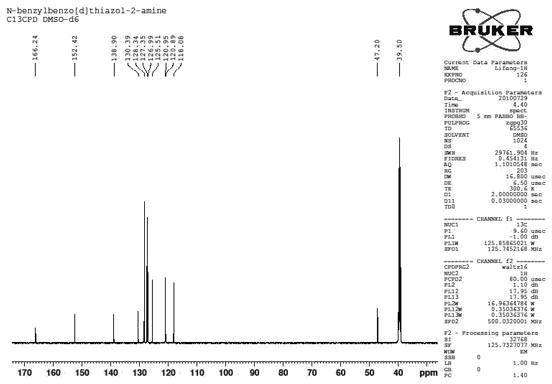
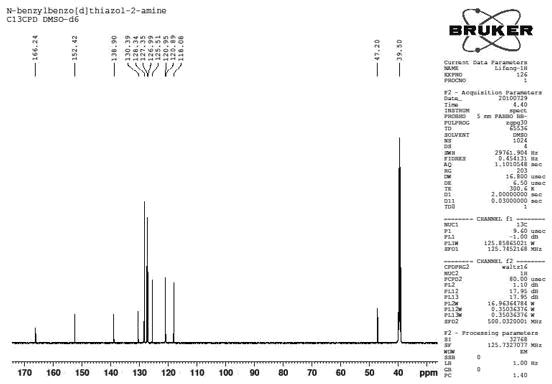
[0034] combine figure 1 , figure 2 , Preparation of N-benzylbenzothiazol-2-amine
[0035] N-benzylbenzo[d]thiazol-2-amine
[0036]
[0037] Under nitrogen protection, 2-aminobenzothiazole (150 mg, 1 mmol), cuprous chloride (1.0 mg, 0.01 mmol, 1 mol%), sodium hydroxide (8.0 mg, 0.2 mmol, 20 mol%), Benzyl alcohol (130 mg, 1.2 mmol, 120 mol%) and p-xylene (1 ml) were added sequentially to a 20 ml Schlenk reaction flask. The reaction mixture was at 130 o After 12 h at C, cool to room temperature. The solvent was removed by rotary evaporation, and then the pure target compound was obtained as a white solid by column chromatography (developing solvent: ethyl acetate / petroleum ether), yield: 96%.
[0038] mp 164.4-165.2 o C; 1 H NMR (500 MHz, DMSO-d 6 ) δ 8.51 (t, J = 5.5 Hz, 1H, NH), 7.67 (d, J = 7.8 Hz, 1H, ArH), 7.39~7.33 (m, 5H, ArH), 7.26 (t, J= 7.1Hz, 1H, ArH), 7.22 (t, J = 7.7 Hz, 1H, ArH), 7.02 (t, J = 7.5 Hz, 1H, ArH), 4.60 (s, 2H, CH 2 N); 13 C NMR (...
Embodiment 2
[0039] Embodiment 2: the preparation of N-(4-methylphenyl) benzothiazol-2-amine
[0040] combine image 3 , Figure 4 , N-(4-methylbenzyl)benzo[d]thiazol-2-amine
[0041]
[0042] Under nitrogen protection, 2-aminobenzothiazole (150 mg, 1 mmol), cuprous chloride (1.0 mg, 0.01 mmol, 1 mol%), sodium hydroxide (8.0 mg, 0.2 mmol, 20 mol%), 4-Methylbenzyl alcohol (146 mg, 1.2 mmol, 120 mol%) and p-xylene (1 ml) were added sequentially to a 20 ml Schlenk reaction flask. The reaction mixture was at 130 o After 12 h at C, cool to room temperature. The solvent was removed by rotary evaporation, and then the pure target compound was obtained as a white solid by column chromatography (developing solvent: ethyl acetate / petroleum ether), yield: 97%.
[0043] mp 188.9-189.4 oC; 1H NMR (500 MHz, DMSO-d6) δ 7.56 (d, J = 7.9 Hz, 1H, ArH), 7.43 (d, J = 8.1Hz, 1H, ArH), 7.29~7.25 (m, 3H, ArH), 7.16 (d, J = 7.8 Hz, 2H, ArH), 7.07 (t, J = 7.5 Hz, 1H, ArH), 6.38 (brs, 1H, NH), 4.58 ...
Embodiment 3
[0044] Embodiment 3: the preparation of N-(4-methoxyphenyl) benzothiazol-2-amine
[0045] N-(4-methoxybenzyl)benzo[d]thiazol-2-amine
[0046]
[0047] Under nitrogen protection, 2-aminobenzothiazole (150 mg, 1 mmol), cuprous chloride (1.0 mg, 0.01 mmol, 1 mol%), sodium hydroxide (165.6 mg, 1.2 mmol, 20 mol%), 4-Methoxybenzyl alcohol (166 mg, 1.2 mmol, 120 mol%) and p-xylene (1 ml) were added sequentially to a 20 ml Schlenk reaction flask. The reaction mixture was at 130 o After 12 h at C, cool to room temperature. The solvent was removed by rotary evaporation, and then the pure target compound was obtained as a white solid by column chromatography (developing solvent: ethyl acetate / petroleum ether), yield: 92%
[0048] mp 173.5-174.0 o C; 1 H NMR (500 MHz, DMSO-d 6 ) 8.42 (t, J = 5.7Hz, 1H), 7.66 (d, J = 7.6 Hz, 1H, ArH), 7.38 (d, J = 8.0 Hz, 1H, ArH), 7.31 (d, J = 7.6 Hz, 1H), 7.21 (t, J = 7.6 Hz, 1H), 6.91 (d, J = 8.5 Hz, 2H, ArH), 4.51 (d, J = 5.6 Hz,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com