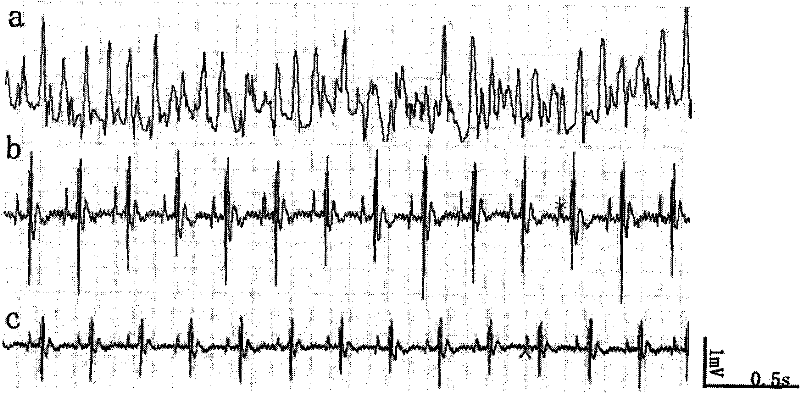
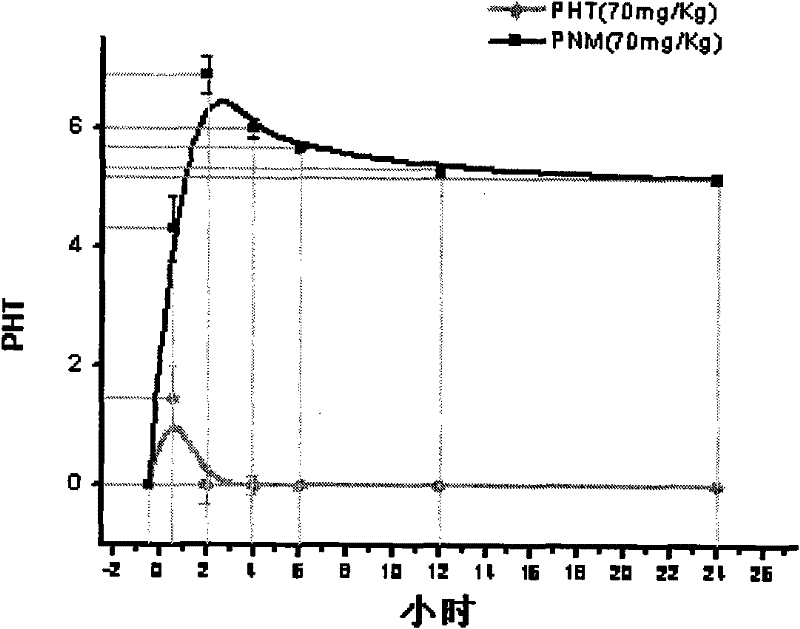
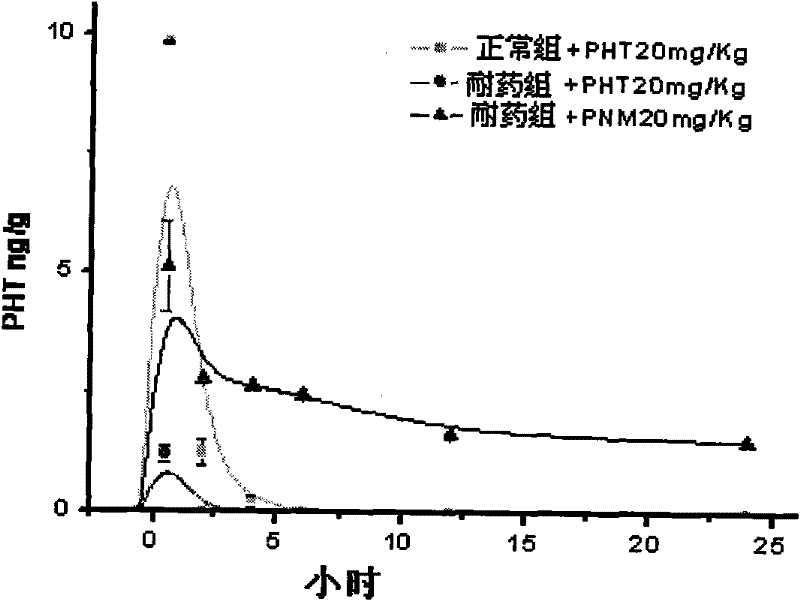
P-glycoprotein monoclonal antibody modified phenytoin targeting nanopreparation and preparation method thereof
A monoclonal antibody and nano-preparation technology, applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, to achieve good biocompatibility, good biocompatibility, and improved safety Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Maleimide group (Mal) capped PEG (5000) 200mg in P 2 o 5 Dry overnight in a vacuum desiccator for purification. Put it into a dried reaction bottle, add 10% stannous octoate toluene solution, seal it and vacuumize at 70°C for 2 hours to remove toluene and oxygen, then add 400 mg of caprolactone, and the temperature rises to 160°C for caprolactone The ring-opening polymerization of the monomer was carried out for 4 hours. After the reaction, cool to room temperature, add dichloromethane to dissolve, and precipitate into ether to remove unreacted caprolactone and the formed caprolactone homopolymer. The methane was dissolved and poured into a large amount of methanol to precipitate. to remove unreacted polyethylene glycol. The purified polymer was dried in a vacuum oven at 40°C for 24 hours, and then stored in a vacuum desiccator. A MAL-PEG-PCL copolymer is obtained.
[0033] Dissolve 20 mg of the polymer and 10 mg of the drug phenytoin in a mixed solution of 1.5 mL...
Embodiment 2
[0036] Maleimide group (Mal) capped PEG (5000) 200mg in P2 o 5 Dry overnight in a vacuum desiccator for purification. Put it into a dried reaction bottle, add 10% stannous octoate toluene solution, seal it and vacuumize it at 70°C for 2 hours to remove toluene and oxygen, then add 800mg of caprolactone, and the temperature rises to 160°C. Bulk ring-opening polymerization, reaction for 4 hours. After the reaction, cool to room temperature, add dichloromethane to dissolve, and precipitate into ether to remove unreacted caprolactone and the formed caprolactone homopolymer. The methane was dissolved and poured into a large amount of methanol to precipitate. to remove unreacted polyethylene glycol. The purified polymer was dried in a vacuum oven at 40°C for 24 hours, and then stored in a vacuum desiccator. A MAL-PEG-PCL copolymer is obtained.
[0037] Dissolve 20 mg of the polymer and 10 mg of the drug phenytoin in a mixed solution of 1.5 mL of dichloromethane and THF, add 10 ...
Embodiment 3
[0040] Maleimide group (Mal) capped PEG (5000) 200mg in P 2 o 5 Dry overnight in a vacuum desiccator for purification. Put it into a dried reaction bottle, add 10% stannous octoate toluene solution, seal it and vacuumize at 70°C for 2 hours to remove toluene and oxygen, then add 1.6g of caprolactone, and the temperature rises to 160°C for caprolactone The ring-opening polymerization of the monomer was carried out for 4 hours. After the reaction, cool to room temperature, add dichloromethane to dissolve, and precipitate into ether to remove unreacted caprolactone and the formed caprolactone homopolymer. The methane was dissolved and poured into a large amount of methanol to precipitate. to remove unreacted polyethylene glycol. The purified polymer was dried in a vacuum oven at 40°C for 24 hours, and then stored in a vacuum desiccator. A MAL-PEG-PCL copolymer is obtained.
[0041] Take 20 mg of the polymer and 10 mg of the drug phenytoin and dissolve them in 1.5 mL of a mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com