Human umbilical mesenchymal stem cell and preparation method thereof
A technique for stem cells and human umbilical cords, which is applied in the field of preparation of primary mesenchymal stem cells, can solve the problems of disputes over the number of MSCs, and achieve the effects of simple and easy operation, good proliferation potential, high purity and high cell activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Preparation of human umbilical cord placental mesenchymal stem cells
[0025] With the informed consent of the puerpera, the umbilical cord or placenta of healthy fetuses delivered at term were collected. Before the umbilical cord or placenta is collected, the puerpera needs to be tested for HIV antibody, hepatitis B virus antibody, hepatitis C virus antibody, treponemal pallidum antibody, alanine aminotransferase, mycoplasma, etc., all of which are qualified to ensure safety before collection.
[0026] 1. After the umbilical cord or placenta is removed from the operating table, immerse in 0.9% saline containing antibiotics and store at 4°C;
[0027] 2. Take out the umbilical cord or placenta in the clean bench, and rinse the residual blood on the surface with D-PBS;
[0028] 3. Cut the umbilical cord or placenta into 1-3mm 3 Put the tissue pieces of different sizes into a 200mL blue cap reagent bottle, add digestion solution with a mass volume percentage c...
Embodiment 2
[0031] Example 2: Cell characteristics of human umbilical cord placental mesenchymal stem cells
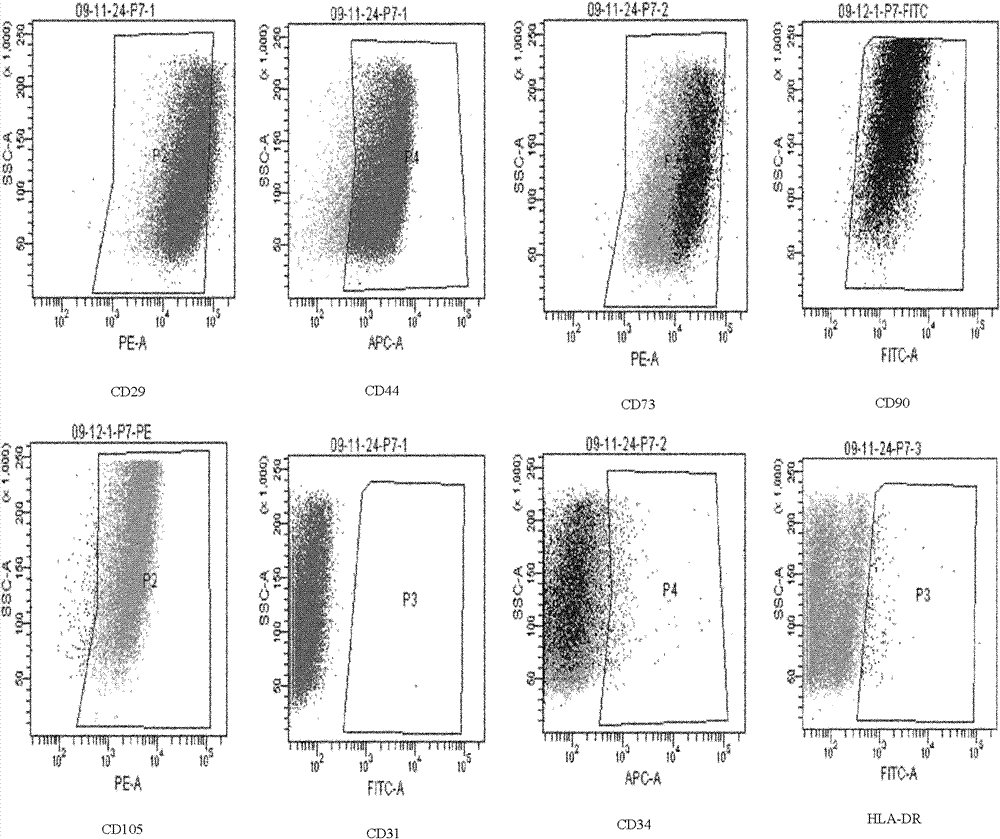
[0032] 1. Immunophenotypic detection of CD29, CD44, CD73, CD90, CD105, CD31, CD34, HLA-DR (see Table 1). Inoculate the mesenchymal stem cells derived from human umbilical cord placenta at passage 4-7 in a roller bottle, digest with 10ml trypsin when the cells reach 80-90% confluence, transfer the cell suspension into a 50ml centrifuge tube and centrifuge at 1000rpm 3 minutes. After centrifugation, the supernatant was discarded, washed twice with PBS, and divided into 1×10 tubes. 6Cells, the first tube is a blank control (only containing mesenchymal stem cells), which is used to adjust the voltage of the flow cytometer, and the second tube is added with an isotype control antibody (PE-MOUSE IgGl / FITC-MOUSE IgG1 / APC-Mouse IgG1κ) 10 μl, add 10 μl of other corresponding antibodies to the other tubes, mix well, incubate at 4°C in the dark for 30 minutes, wash once with PBS, discard t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com