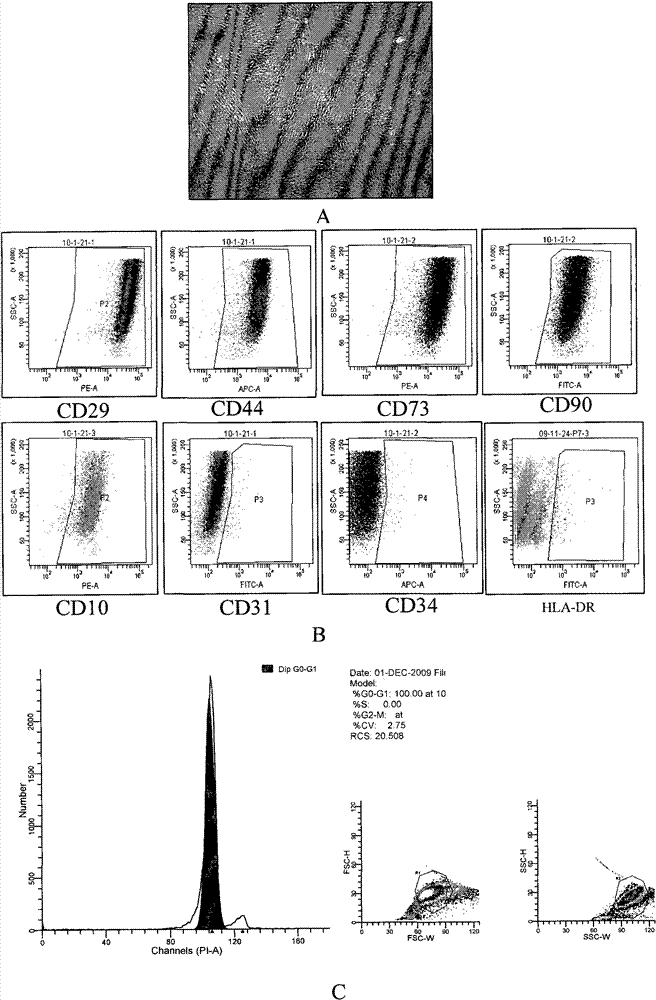
Method for amplifying mesenchymal stem cells of human umbilical cord and placenta in vitro
An in vitro expansion and stem cell technology, applied in animal cells, vertebrate cells, bone/connective tissue cells, etc., can solve the adverse reactions of patients, the roller bottle operation is not very convenient, and is not suitable for large-scale expansion, etc. problems, achieve good proliferation potential, simple operation, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation of human umbilical cord placental mesenchymal stem cells:
[0030] With the informed consent of the parturient, the umbilical cord or placenta of healthy fetuses delivered by cesarean section was collected. Before collecting the umbilical cord or placenta, women need to be tested for HIV antibody, hepatitis B virus antibody, hepatitis C virus antibody, Treponema pallidum antibody, alanine aminotransferase, and mycoplasma, etc., and they can only be collected after all qualified to ensure safety.
[0031] 1. After the umbilical cord or placenta is removed from the operating table, immerse it in 0.9% normal saline containing antibiotics and store at 4°C;
[0032] 2. Take out the umbilical cord or placenta from the cleaning table, and rinse the residual blood on the surface with D-PBS;
[0033] 3. Cut the umbilical cord or placenta into 1mm 3 Put the large and small tissue block into a 200mL blue cap reagent bottle, add 30mL of type II collagenase with a mass / volume ra...
Embodiment 2
[0037] MesenPRO RS TM Optimization of Medium culture system
[0038] MesenPRO RS as the best culture system for in vitro culture of human umbilical cord placental mesenchymal stem cells TM Medium, considering the following factors, we supplement the original medium with a final concentration of 10%-15% FBS, 2mM L-glutamine and gentamicin sulfate: (1) Fetal bovine serum is rich in cell growth The necessary nutrients. Its functions after cell seeding are roughly as follows. ①Protect cells from damage; ②Promote cell adhesion; ③Promote cell proliferation: A variety of growth factors, especially platelet growth-promoting factors, can promote cell division and are the main factors that promote cell proliferation. In addition, insulin in serum can promote the uptake of glucose and amino acids by cells, which is related to cell division; insulin-like growth factor, which can bind to the insulin receptor expressed by cells, and thus has the same effect as insulin; growth-promoting horm...
Embodiment 3
[0045] Large-scale expansion of human umbilical cord placental mesenchymal stem cells under optimal roller bottle culture conditions
[0046] 1. Use MesenPRO RS TM When the cell density of the mesenchymal stem cells cultured in the Medium culture system reaches 80%-90%, trypsin digestion to prepare a single cell suspension;
[0047] 2. Inoculate the suspension on a surface area of 850cm 2 In the roller bottle, the total number of cells is 1×10 6 (1400 cells / cm 2 ), join MesenPRO RS TM Medium culture medium to 100ml, shake the suspension vertically and then slowly rotate the roller bottle horizontally for 1 week. The purpose is to evenly distribute the cells in the roller bottle, and then place the roller bottle in INCUDRIVE D-I CO 2 In the incubator rotating system, in order to stabilize the optimal temperature, the roller bottle should be located behind the incubator as much as possible and driven at 4 positions with a wide axial distance;
[0048] 3. At 37℃, rotate for 2 hours a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
| surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com