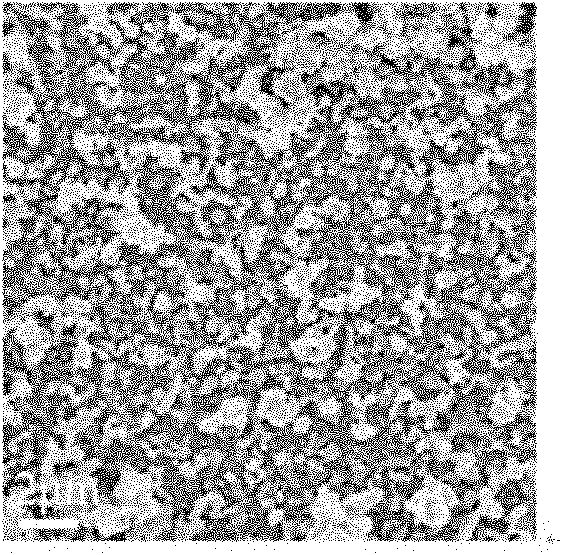
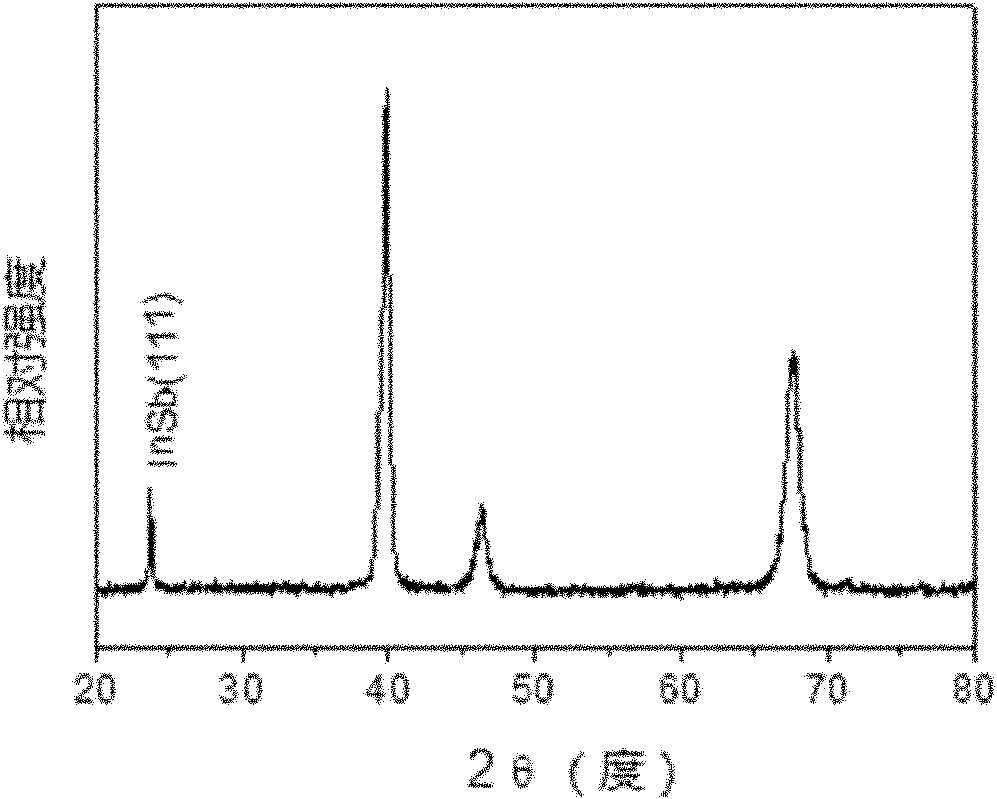
Method for preparing super-hydrophobic indium antimonide film by deposition in ionic liquid
A super-hydrophobic, ionic liquid technology, applied in the micro-nano field, can solve the problems of demand, harsh deposition conditions, strict preparation conditions, etc., and achieve the effect of simple and easy method and remarkable hydrophobic performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] ① Electrochemical deposition:
[0021] Weigh 0.1383 grams of anhydrous indium chloride and 0.1427 grams of anhydrous antimony chloride, dissolve in 25 milliliters of ionic liquid 1-methyl-3-ethylimidazole bistrifluoromethylsulfonylimide salt, and prepare a concentration of 25mM solution.
[0022] Put the platinum-coated glass substrate into acetone for ultrasonic cleaning, then take it out and dry it for use.
[0023] Immerse the cleaned substrate in the above-mentioned ionic liquid solution, use a silver electrode as a reference electrode, and a platinum electrode as an auxiliary electrode, perform electrochemical deposition at a voltage of -1.25V, take it out after 20 hours, and clean it with acetone and deionized water. After drying, a deposited film was obtained.
[0024] ② Chemical modification of film surface:
[0025] The deposited film was immersed in an ethanol solution of 1-decyl phosphoric acid with a concentration of 10 mM, kept for 2 hours, taken out, wa...
Embodiment 2
[0030] ① Electrochemical deposition:
[0031] Weigh 0.0553 gram of anhydrous indium chloride and 0.0571 gram of anhydrous antimony chloride, dissolve them in 25 milliliters of ionic liquid 1-methyl-3-ethylimidazole bistrifluoromethylsulfonylimide salt, and prepare a concentration of 10mM solution.
[0032] The conductive glass substrate was ultrasonically cleaned in acetone and then taken out to dry for use.
[0033] Immerse the cleaned substrate in the above-mentioned ionic liquid solution, use the silver electrode as the reference electrode, and the platinum electrode as the auxiliary electrode, carry out electrochemical deposition at a voltage of -1.35V, take it out after 6 hours, and wash it with acetone and deionized water. After drying, a deposited film was obtained.
[0034] ② Chemical modification of film surface:
[0035] The deposited film was immersed in an ethanol solution of 1-tetradecylphosphoric acid with a concentration of 10 mM, kept for 2 hours, taken out,...
Embodiment 3
[0037] ① Electrochemical deposition:
[0038] Weigh 0.2766 grams of anhydrous indium chloride and 0.2854 grams of anhydrous antimony chloride, dissolve them in 25 milliliters of ionic liquid 1-methyl-3-ethylimidazole bistrifluoromethylsulfonylimide salt, and prepare a concentration of 50mM solution.
[0039] The copper substrate was ultrasonically cleaned in acetone and then taken out to dry for use.
[0040] The cleaned substrate was immersed in the above-mentioned ionic liquid solution, with a silver electrode as a reference electrode and a platinum electrode as an auxiliary electrode, and electrochemical deposition was carried out at a voltage of -1.45V. After 4 hours, it was taken out and cleaned with acetone and deionized water. After drying, a deposited film was obtained.
[0041] ② Chemical modification of film surface:
[0042] The deposited film was immersed in a n-hexane solution of perfluorooctyltrichlorosilane with a concentration of 2mM, kept for 2 hours, taken...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com