Catalyst for degrading lignin, catalyst for degrading aromatic hydrocarbon, and porphyrin
A technology of lignin and catalyst, applied in physical/chemical process catalyst, organic compound/hydride/coordination complex catalyst, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
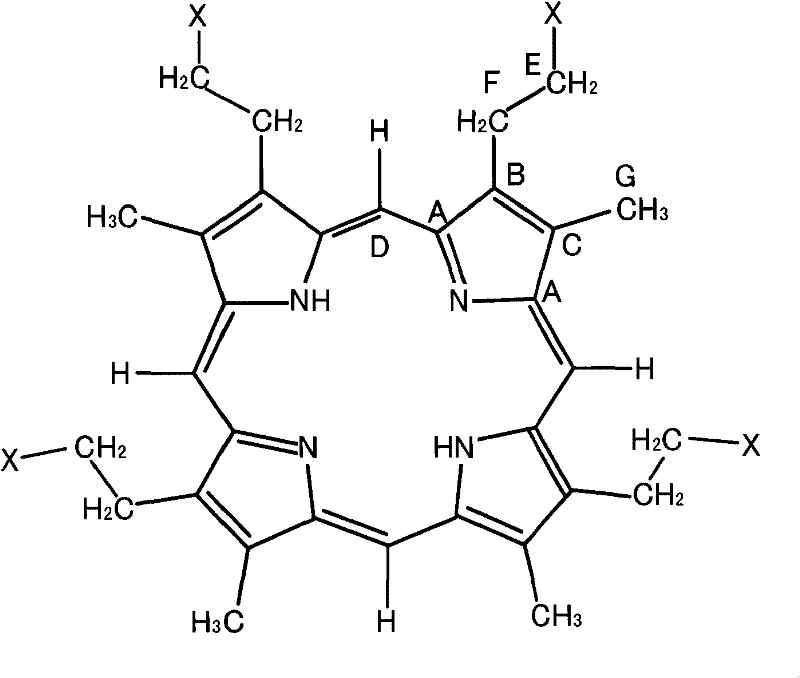
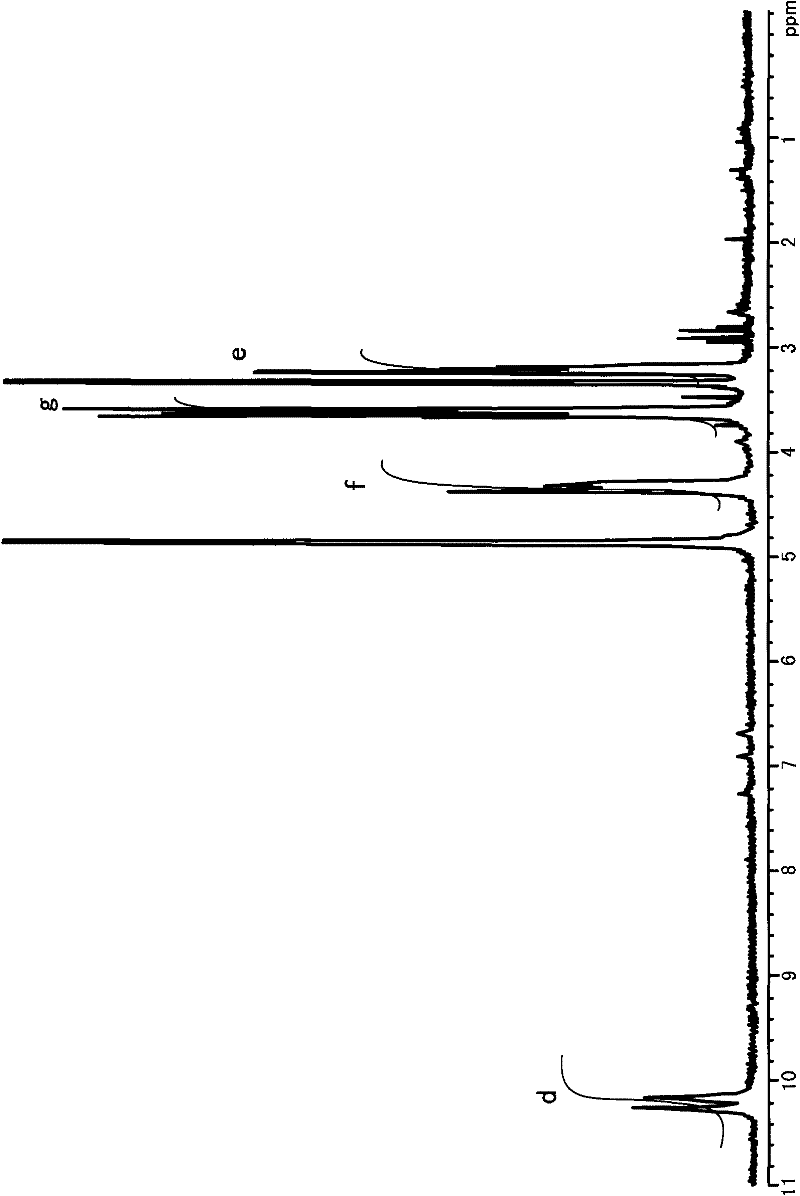
[0099] As described above, alcohols and organic acids can be produced by adding porphyrin (pyrrole compound) to lignin as a lignin decomposition catalyst, and further decomposition products can be obtained by photolysis of lignin, and the lignin released from lignin can also be separated. Hydrogen ion. Furthermore, tetrapyrrole compounds and synthesized porphyrins each having a porphyrin structure are effective as catalysts for decomposing aromatic hydrocarbons such as dioxins. In this case, the porphyrin usable herein may be, for example, a porphyrin biologically produced using Escherichia coli. In this Preparation Example, the preparation of the pyrrole compound using Escherichia coli will be described.
[0100] Cells (JD23504 obtained from National Biological-Resources) that had been inserted with the transposon-derived transposon of Escherichia coli were cultured at 37° C. in 2 ml of LB medium (bacteria-tryptone: 1 %; bacteria-yeast extract: 0.5%; NaCl: 0.5%) for 12 hour...
Embodiment 1
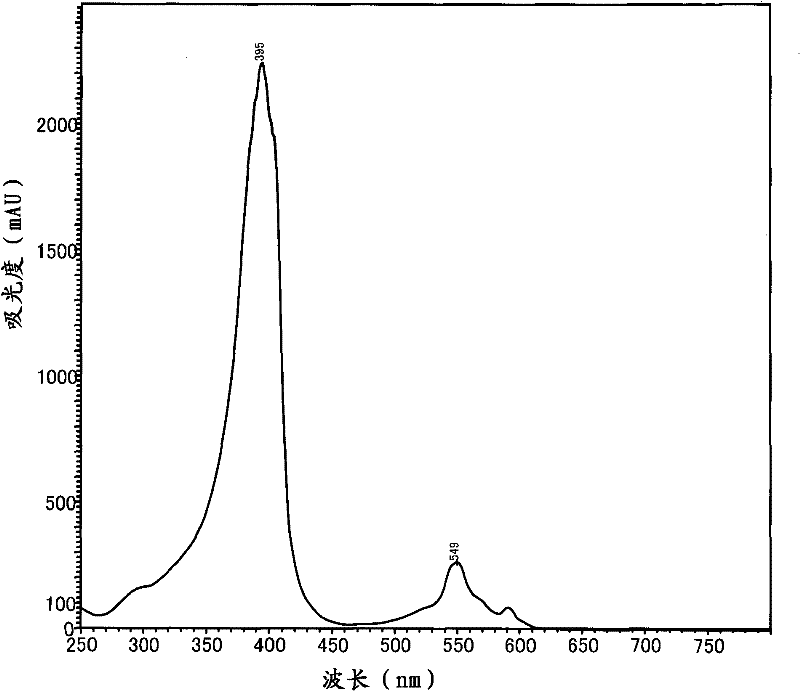
[0156] In this example, the lignin used was a high-purity product (obtained from Sigma, Cat. No. 471003 with a molecular weight of 60,000) without any impurities such as reducing sugars and cellulose. 1 ml of a solution containing 2.5 mg / ml of this lignin, 0.05 M KOH and 50 μg / ml of the porphyrin with 4 carboxyl groups in the molecule prepared in the aforementioned Preparation Example 1 was introduced into an Eppendorf polypropylene tube almost similar to A light-transmitting cylindrical tube, and the contents of the tube were irradiated for 12 hours with UV light emitted by a UV lamp of the ENF type obtained from the company SPECTRONICS. Then, the tube was heated to 80° C. for 60 minutes, and the gas evaporated from the tube contents was analyzed using a GC / MS QP-2010 from Shimadzu Corporation equipped with a column DB-WAX, and detected 170 μg / ml of methanol. At this time, 140 μg / ml of formic acid, 25 μg / ml of malic acid, 19 μg / ml of acetic acid, 5.4 μg / ml of succinic acid, a...
Embodiment 2
[0161] The lignin used in this example is a high-purity product (obtained from Sigma, Cat. No. 471003, molecular weight: 60,000) without any impurities (such as reducing sugar and cellulose). 1 ml of this lignin containing 2.5 mg / ml, 0.05 M KOH and, as synthetic porphyrin, 50 μg / mL protoporphyrin IX (obtained from ALDRICH company) with 2 carboxyl groups in each molecule and A solution of uroporphyrin I with 8 carboxyl groups (from Sigma) or coproporphyrin I with 4 carboxyl groups in the molecule (from ALDRICH) was introduced into a cylinder with light transmission almost similar to Eppendorf polypropylene tubes shaped tubes, and the contents of the tubes were irradiated for 12 hours with UV light emitted by an ENF type UV lamp obtained from the company SPECTRONICS. Then, the reaction liquid thus obtained was heated to 80° C. for 60 minutes, and the gas evaporated from the contents of the tube was analyzed using GC / MSQP-2010 equipped with column DB-WAX obtained from Shimadzu Co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Resonant frequency | aaaaa | aaaaa |
| Resonant frequency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com