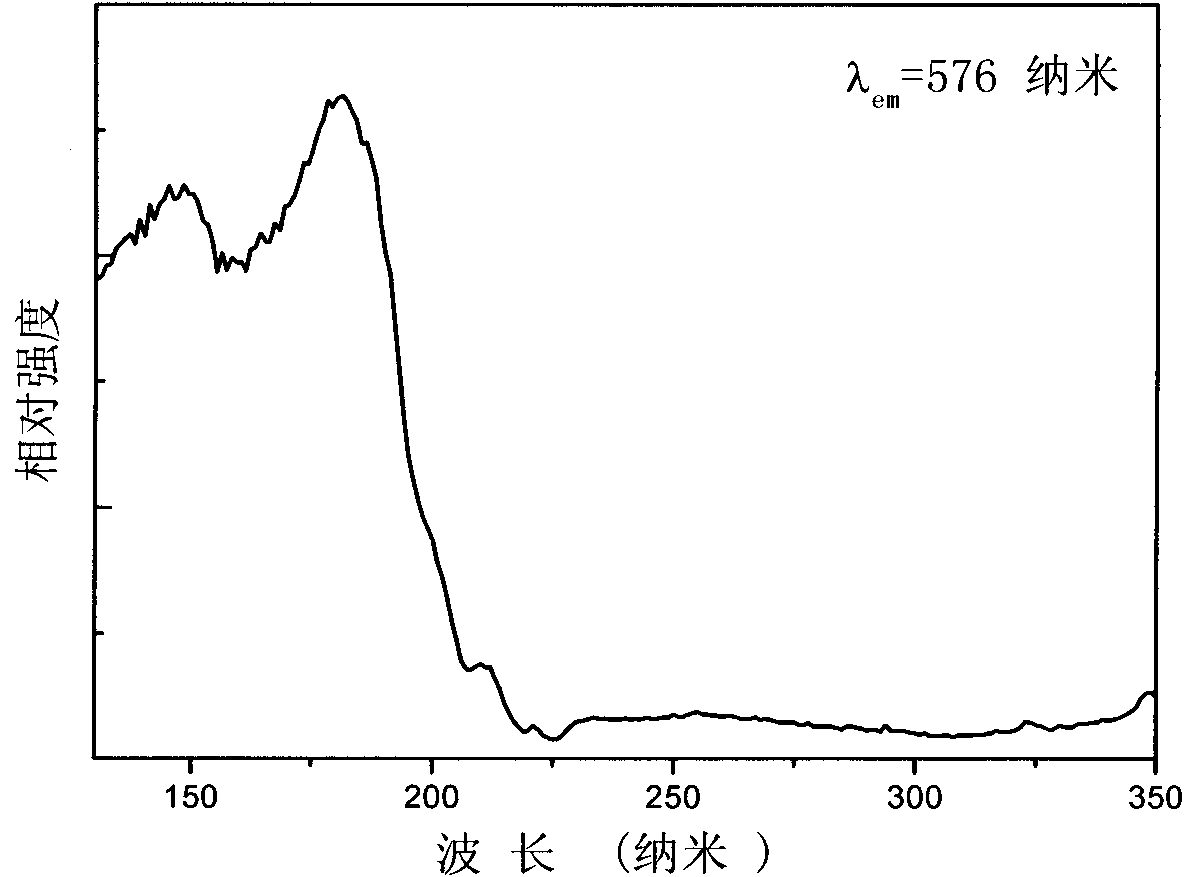
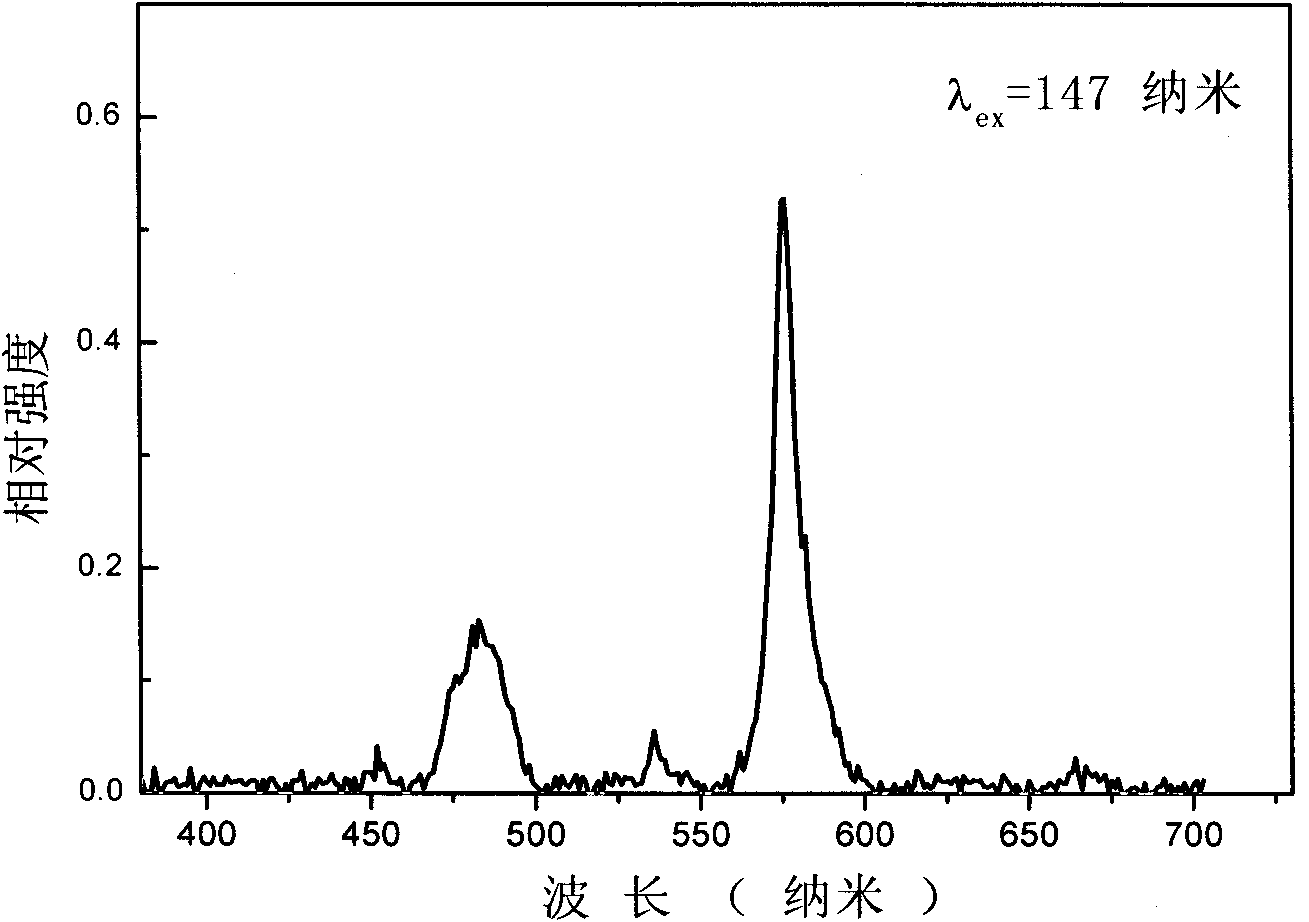
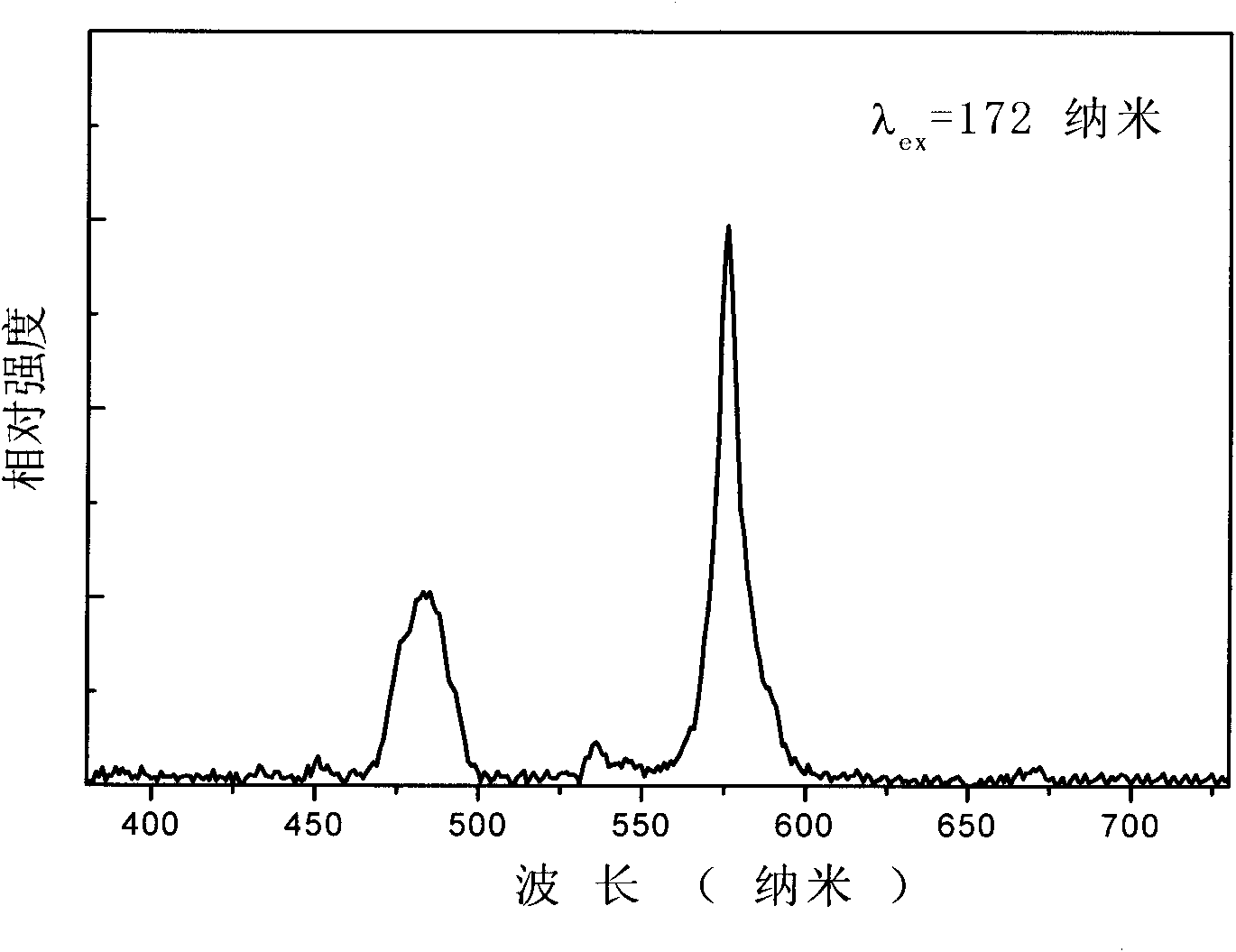
Borofluoride white light emitting material and preparation method thereof
A luminescent material, fluoroborate technology, applied in luminescent materials, chemical instruments and methods, climate sustainability, etc., can solve the problems of heavy metal mercury pollution of mercury-containing fluorescent lamps, achieve low price, large output, and simple preparation method Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: composition is Ca 4.98 (BO 3 ) 3 F: Dy 0.01 , Na 0.01 White phosphor
[0028] Weigh calcium carbonate (CaCO 3 ) 0.9969 g, sodium carbonate (Na 2 CO 3 ) 0.0011 g, boric acid (H 3 BO 3 ) 0.3710 g, dysprosium oxide (Dy 2 o 3 ) 0.0037 g, ammonium fluoride (NH 4 F) 0.0889 g, fully ground in an agate mortar and mixed evenly, sintered at 700° C. for 12 hours, took out the sample and ground it, and finally obtained the product.
Embodiment 2
[0029] Embodiment 2: composition is Sr 4.98 (BO 3 ) 3 F: Dy 0.01 , K 0.01 White phosphor
[0030] Weigh strontium carbonate (SrCO 3 ) 1.4704 g, potassium carbonate (K 2 CO 3 ) 0.0014 grams, boron trioxide (B 2 o 3 ) 0.2088 g, dysprosium oxide (Dy 2 o 3 ) 0.0037 g, ammonium fluoride (NH 4 F) 0.0889 g, fully ground in an agate mortar and mixed evenly, sintered at 500° C. for 24 hours, took out the sample and ground it, and finally obtained the product.
Embodiment 3
[0031] Embodiment 3: composition is Sr 4.6 (BO 3 ) 3 F: Dy 0.2 , Na 0.2 White phosphor
[0032] Weigh strontium nitrate (Sr(NO 3 ) 2) 1.9470 g, sodium carbonate (Na 2 CO 3 ) 0.0223 g, boric acid (H 3 BO 3 ) 0.3710 g, dysprosium oxide (Dy 2 o 3 ) 0.0746 g, ammonium fluoride (NH 4 F) 0.0889 g, fully ground in an agate mortar and mixed evenly, sintered at 900° C. for 6 hours, took out the sample and ground it, and finally obtained the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com