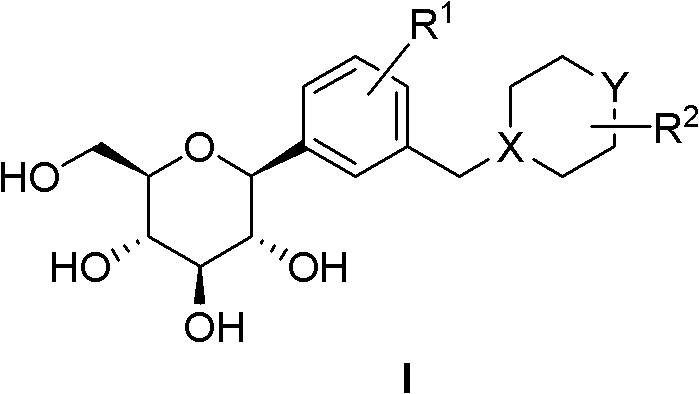
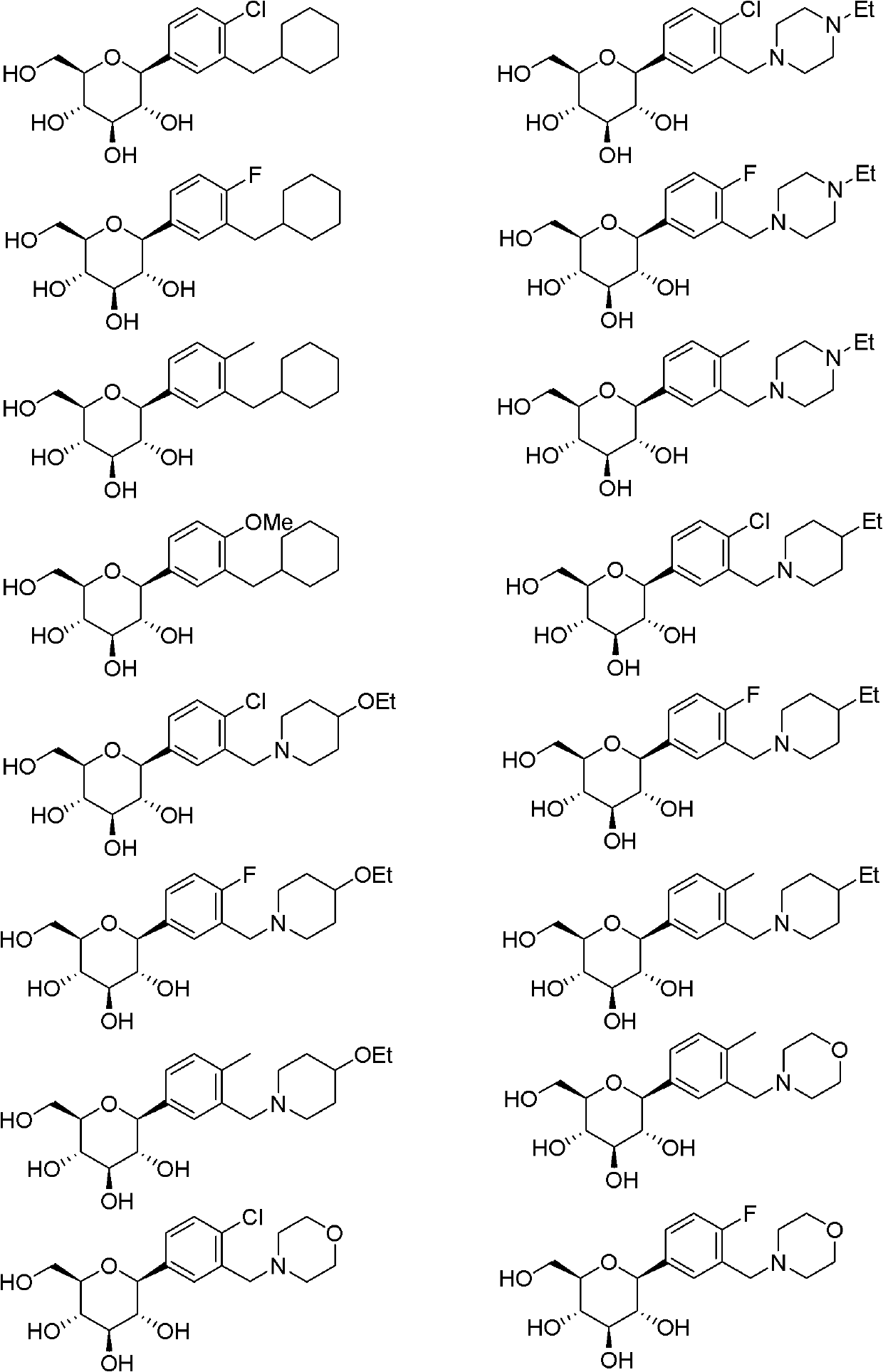
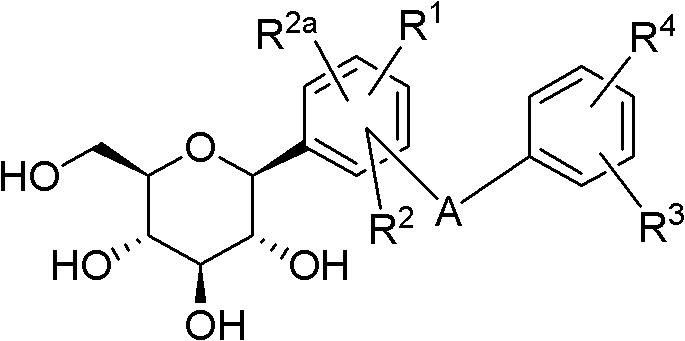
C-glucoside derivatives containing saturated six-membered ring as well as preparation method and application thereof
A C1-C3 compound technology, applied in the field of type 2 sodium glucose co-transporter inhibitors and its preparation, can solve problems such as weight gain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] 1-[4-Chloro-3-(cyclohexylmethyl)phenyl]-1-deoxy-β-D-glucopyranose
[0077]
[0078] A. 4-Bromo-1-chloro-2-(cyclohexylmethyl)benzene
[0079] Add 2.19g (10mmol) of 2-chloro-5-bromobenzaldehyde and 10mL of anhydrous tetrahydrofuran into a 100mL round-bottomed flask, cool the solution with an ice-water bath and stir it electromagnetically, and add 11mL (11mmol) dropwise with a constant pressure dropping funnel; 1.0 M) a THF solution of cyclohexylmagnesium bromide. After the dropwise addition, the reaction mixture was stirred at room temperature for one hour and poured carefully into 200 mL of ice water. The pH was adjusted to 3-4 with concentrated hydrochloric acid. The resulting acidic system was extracted twice with 100 mL of dichloromethane, the combined extracts were washed once with saturated brine, dried over anhydrous sodium sulfate, and evaporated to dryness on a rotary evaporator to obtain a colorless oily substance (5-bromo- 2-Chlorophenyl)(cyclohexyl)methano...
Embodiment 2-10
[0089] It can be understood that, using the method and process of Example 1, changing R 1 , R 2 The compounds listed in the table below can be obtained.
[0090]
[0091]
[0092]
Embodiment 14
[0094] 1-{4-Chloro-3-[(4-ethylpiperazin-1-yl)methyl]phenyl}-1-deoxy-β-D-glucopyranose
[0095]
[0096] A. 4-Bromo-1-chloro-2-[(4-ethylpiperazin-1-yl)methyl]benzene
[0097]Add 2.84g (10mmol) 5-bromo-2-chlorobenzyl bromide, 1.14g (10mmol) 1-ethylpiperazine, 1.01g (10mmol) triethylamine and 20mL anhydrous tetrahydrofuran to a 100mL round bottom flask , the resulting reaction mixture was stirred overnight at room temperature and then poured into 200 mL of water, extracted twice with 100 mL of dichloromethane, the combined extraction liquid was washed once with saturated brine, dried over anhydrous sodium sulfate, and evaporated to dryness on a rotary evaporator to obtain Off-white solid, 4-bromo-1-chloro-2-[(4-ethylpiperazin-1-yl)methyl]benzene. 3.11g, yield 98%, ESI-MS, m / z=317.2 ([M( 79 Br)+1]), 319.3([M( 81 Br)+1]).
[0098] B. 1-{4-chloro-3-[(4-ethylpiperazin-1-yl)methyl]phenyl}-1-deoxy-β-D-glucopyranose
[0099] In a 100mL round bottom flask, add 3.11g (9.8mmol) of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com