Fluorinated terphenyl liquid crystal compound containing two 3-butenyls and preparation method thereof
A technology of liquid crystal compounds and terphenyls, which is applied in chemical instruments and methods, liquid crystal materials, organic chemistry, etc., can solve problems such as poor stability of ultraviolet light, large birefringence, and unstable water, and achieve large birefringence , high-definition highlights, the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
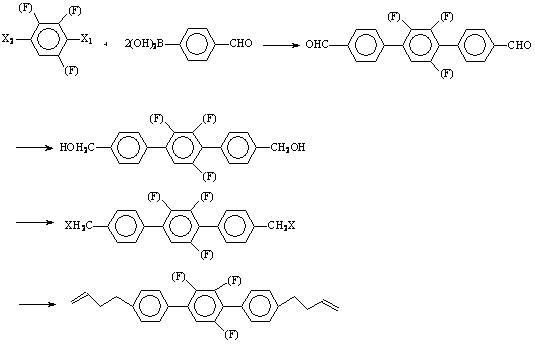
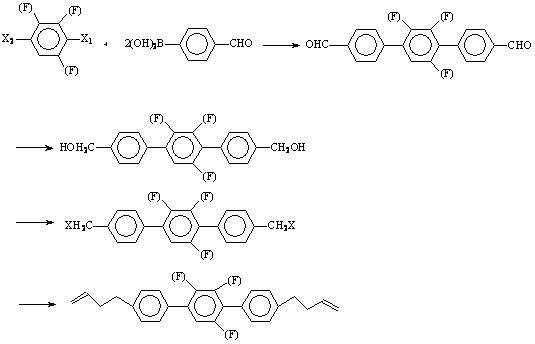
[0040] Preparation of 4,4''-bis(3-butenyl)-3',5'-difluoro-(1,1',4',1'')terphenyl.
[0041] step 1 Preparation of 4,4''-bisformyl-3',5'-difluoro-(1,1',4',1'')terphenyl:
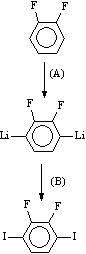
[0042] Add 36.6g of 2,6-difluoro-1,4-diiodobenzene, 36g of p-formylphenylboronic acid, 300ml of toluene, 200ml of ethanol, 200ml of water into a 1000ml three-necked flask, replace the air with nitrogen, and add tetrakistriphenylphosphine palladium 1.6 g, heated to reflux with stirring for 6 hours.
[0043] Remove the inorganic salts in the reaction solution by filtration while it is hot, wash the filter cake with hot toluene, separate the filtrate to remove the water layer, wash the organic layer with hot water, and freeze it in the refrigerator. The precipitated crystals were filtered to obtain 24.6 g of pale yellow crystals.
[0044] step 2 Preparation of 4,4''-bishydroxymethyl-3',5'-difluoro-(1,1',4',1'')terphenyl
[0045] Add 24.6g of 4,4''-bisformyl-3',5'-difluoro-(1,1',4',1'') terphenyl and 400ml...
Embodiment 2
[0057] Preparation of 4,4''-bis(3-butenyl)-2',3'-difluoro-(1,1',4',1'')terphenyl
[0058] Referring to the preparation method similar to Example 1, the initial raw material is coupled with p-formylphenylboronic acid and 2,3-difluoro-1,4-diiodobenzene, and after four steps of reaction as in Example 1, it can be prepared 4,4''-bis(3-butenyl)-2',3'-difluoro-(1,1',4',1'')terphenyl
Embodiment 3
[0060] Preparation of 4,4''-bis(3-butenyl)-3',6'-difluoro-(1,1',4',1'')terphenyl
[0061] Referring to the preparation method similar to Example 1, the initial raw material is coupled with p-formylphenylboronic acid and 2,5-difluoro-1,4-diiodobenzene, and after four steps of reaction as in Example 1, it can be prepared 4,4''-Bis(3-butenyl)-3',6'-difluoro-(1,1',4',1'')terphenyl is obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com