Method for preparing dipivefrine
A technology of dipifolin and chloroacetyl catechol is applied in the preparation of organic compounds, chemical instruments and methods, preparation of aminohydroxy compounds, etc., and can solve the difficulty of post-processing, many side reactions, and large consumption of pivaloyl chloride and other problems, to achieve the effects of simple operation and post-treatment, mild reaction conditions and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
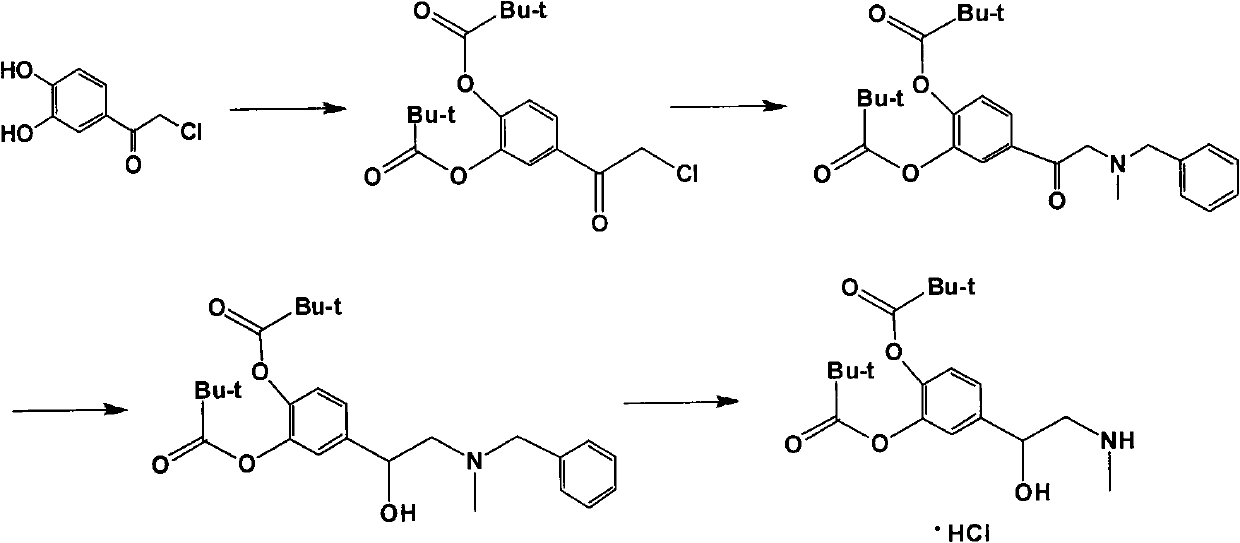
[0023] Embodiment 1: the preparation of dipephrine
[0024] Add 600g (3.21mol) of 4-chloroacetylcatechol and 6L of dichloromethane into a 10L four-neck flask, cool the system down to 5°C, add 666g (6.58mol) of triethylamine, and then dropwise add 784g (6.5mol) ) pivaloyl chloride, continue to stir for 3h after the dropwise addition. Suction filtration and rotary evaporation of the filtrate; 990 g of a yellow-brown solid, namely phenyl 4-(2-chloroacetyl)-1,2-dipivalate, with a content of 96.2% was obtained.
[0025] Add 526g (4.35mol) of N-methylbenzylamine, 370g (3.66mol) of triethylamine, 25g (0.15mol) of KI, and 3L of DMF into a 10L four-necked flask. The temperature was lowered to 0°C, and a DMF solution of 990 g (2.8 mol) of 4-(2-chloroacetyl)-1,2-dipivaloic acid phenyl ester was added dropwise for 2 h. Stir at room temperature for 4h.
[0026] Suction filtration, add 10L water to the filtrate to wash 3 times, separate the organic phase, and rotary evaporate the organic...
Embodiment 2
[0034] Embodiment 2: the preparation of dipephrine
[0035] Add 600g (3.21mol) of 4-chloroacetylcatechol and 6L of dichloromethane into a 10L four-neck flask, cool the system down to 10°C, add 666g (6.58mol) of triethylamine, and then dropwise add 784g (6.5mol) ) pivaloyl chloride, continue to stir for 3h after the dropwise addition. Suction filtration, rotary distillation of the filtrate; 978.2 g of a yellow-brown solid, namely phenyl 4-(2-chloroacetyl)-1,2-dipivalate, with a content of 96.2% was obtained.
[0036] Add 526g (4.35mol) of N-methylbenzylamine, 370g (3.66mol) of triethylamine, 25g (0.15mol) of KI, and 3L of DMF into a 10L four-necked flask. The temperature was lowered to 0° C., and a DMF solution of 978.2 g (2.77 mol) of 4-(2-chloroacetyl)-1,2-dipivaloic acid phenyl ester was added dropwise for 2 h. Stir at room temperature for 4h.
[0037] Suction filtration, add 10L water to the filtrate and wash 3 times, separate the organic phase, and rotary evaporate the ...
Embodiment 3
[0042] Embodiment 3: the preparation of dipephrine
[0043] Add 600g (3.21mol) of 4-chloroacetylcatechol and 6L of dichloromethane into a 10L four-necked flask, cool the system down to 5°C, add 897g (6.5mol) of potassium carbonate, and then dropwise add 784g (6.5mol) Pivaloyl chloride, continue to stir for 5h after the dropwise addition. Suction filtration and rotary evaporation of the filtrate; 900 g of a yellow-brown solid, namely phenyl 4-(2-chloroacetyl)-1,2-dipivalate, with a content of 95.6% was obtained.
[0044] Add 526g (4.35mol) of N-methylbenzylamine, 414g (3.0mol) of potassium carbonate, 25g (0.15mol) of KI, and 3L of DMF into a 10L four-necked flask. The temperature was lowered to 0° C., and a DMF solution of 900 g (2.55 mol) 4-(2-chloroacetyl)-1,2-dipivalic acid phenyl ester was added dropwise for 3 h. Stir at room temperature for 6h.
[0045] Suction filtration, add 10L water to the filtrate and wash 3 times, separate the organic phase, and rotary evaporate t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com