Triazolopyrimidine compound and preparation method thereof
A technology for azolopyrimidines and compounds is applied in the field of preparation of triazolopyrimidine compounds and can solve problems such as low yield of active components
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
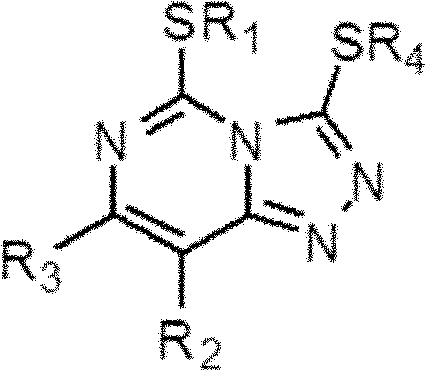
[0041] According to the second aspect of the present invention, the present invention also provides a method for preparing a triazolopyrimidine compound having a general formula as shown in formula (2), the method comprising: under cyclization reaction conditions, an organic base and Under the existence of hydrogen peroxide, make the compound and carbon disulfide contact reaction of general formula shown in formula (3);
[0042]
[0043] Formula (2)
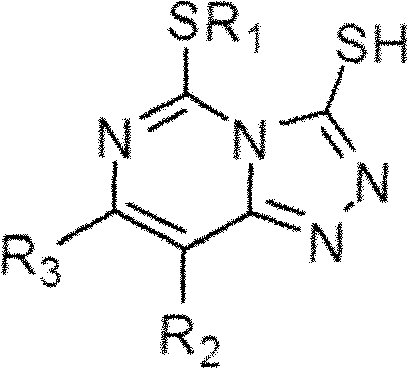
[0044] Among them, R 1 for C 1 -C 4 the alkyl group; R 2 and R 3 are the same or different, and each independently selected from hydrogen, halogen, C 1 -C 4 Alkyl or -OR 5 , where R 5 for C 1 -C 4 of alkyl. Preferably, R 1 is methyl or ethyl; R 2 and R 3 are the same or different, and are each independently selected from fluorine, chlorine, methyl, ethyl, methoxy or ethoxy. Further preferably, R 1 is methyl or ethyl; R 2 and R 3 one of which is fluorine or chlorine and the other is hydrogen, methyl or ethyl; ...
Embodiment 1
[0075] This example is used to illustrate the triazolopyrimidine compound and its preparation method provided by the present invention.
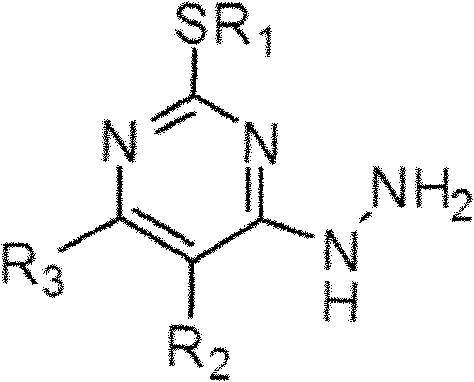
[0076] Add 166g of 2,4-dichloro-5-fluoropyrimidine (purchased from Chengdu Yuyang High-tech Development Co., Ltd.) and 154g of sodium methyl mercaptide to a 1L flask, and react at 70°C for 6 hours. Then, 237 g of methanol and 294 g of hydrazine hydrate were added thereto, and reacted at 70° C. for 7 hours. The resulting mixture was cooled to 5 °C and the solid was collected by vacuum filtration, washed with 150 mL of cold methanol, and dried to a stable weight to give 149 g of a white solid material with a melting point of 146 °C, NMR data Such as: 1 H-NMR (500MHz DMSO) δ: 2.55 (s, 3H), 4.71 (d, 2H), 8.15 (s, 1H), 8.45 (t, 1H); mass spectrometry data such as: MS (m / z) 175 ( +H + ), 197 (+Na + ). From this, it was confirmed that the solid substance was 5-fluoro-4-hydrazino-2-methylthiopyrimidine, its purity was 98% by weight, and its yie...
Embodiment 2
[0080] This example is used to illustrate the triazolopyrimidine compound and its preparation method provided by the present invention.
[0081] Add 182.5g of 2,4,5-trichloropyrimidine (purchased from Shanghai Yitai Technology Co., Ltd.) and 185g of sodium ethanethiolate into a 1L flask, contact and react at 100°C for 10 hours, and then add 237 g of methanol and 294 g of hydrazine hydrate were added and reacted at 70° C. for 7 hours. The resulting mixture was cooled to 5 °C and the solid was collected by vacuum filtration, washed with 150 mL of cold methanol, and dried to a stable weight to give 191 g of a white solid material with a melting point of 120 °C, NMR data Such as: 1 H-NMR (500MHz DMSO) δ: 1.32 (t, 3H), δ: 2.98 (dd, 2H), 4.64 (d, 2H), 7.77 (s, 1H), 8.69 (t, 1H); mass spectrometry data such as : MS(m / z)205(+H + ), 227(+Na + ). From this, it was confirmed that the solid substance was 5-chloro-4-hydrazino-2-ethylthiopyrimidine, its purity was 98% by weight, and it...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com