Vectors for delivery of light-sensitive proteins and methods of use
A light-sensitive protein and carrier technology, applied in various fields, can solve the unresolved problems of targeting and delivery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0174] Example 1: Injection method
[0175] Operated on All procedures for animals.
[0176] For intravitreal injection, mice were anesthetized with ketamine (72 mg / kg) / xylazine (4 mg / kg) by intraperitoneal injection. After anesthesia, a Hamilton syringe fitted with a 33-gauge, flat-ended needle was used. The needle passes through the sclera and enters the vitreous cavity near the limbus at the equator. Injections were performed with direct visualization of the needle in the middle of the vitreous cavity. The total volume delivered was 1.5 μl, containing the different concentrations of the AAV vectors tested.
[0177] For subretinal injections, the eyes of mice were dilated with 1% atropine eye drops followed by topical administration of 2.5% phenylephrine 1 hour before anesthesia. Mice were then anesthetized with ketamine (72 mg / kg) / xylazine (4 mg / kg) by intraperitoneal injection. A 30 1 / 2 gauge disposable needle was passed through the cornea to create a small hole in...
Embodiment 2
[0179] Example 2: Screening of AAV serotypes 1, 2, 5, 7, 8 and 9 for transduction of retinal bipolar cells
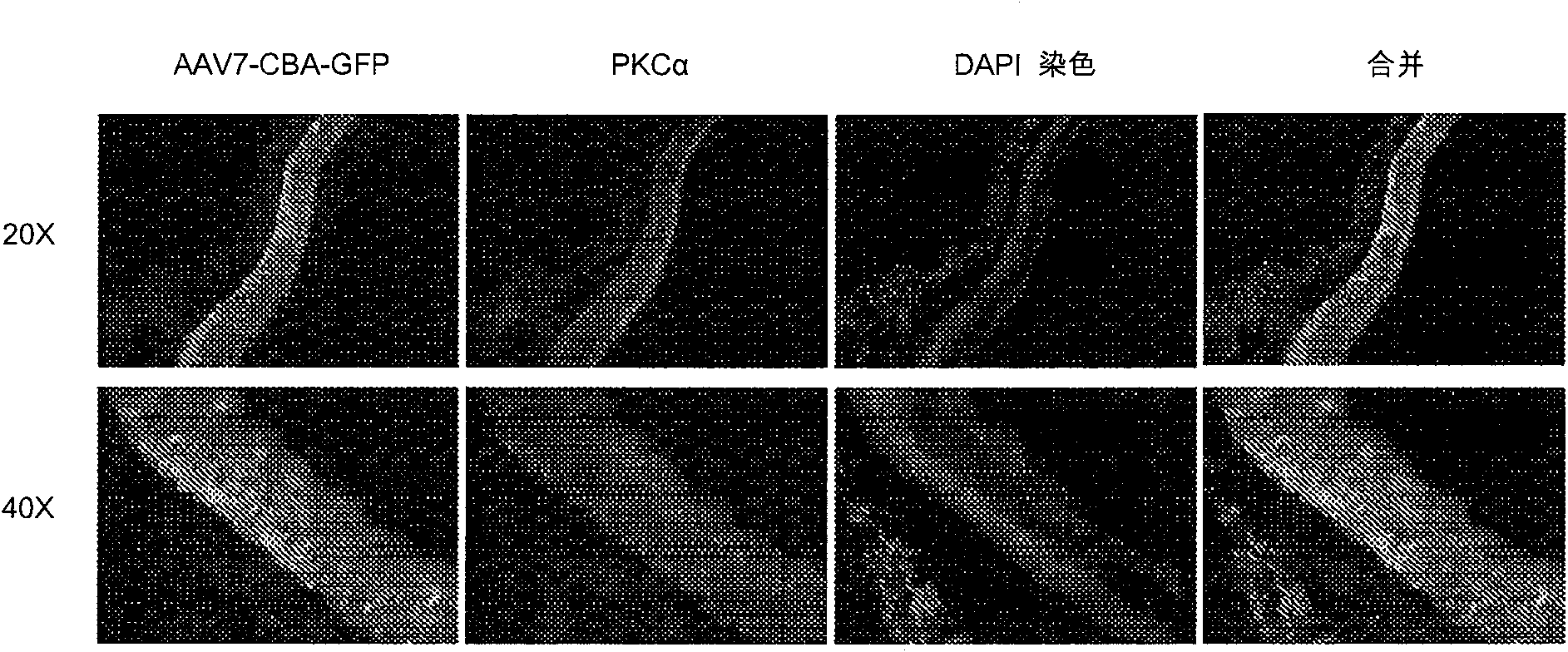
[0180] This example performs a screening of known and characterized viral vectors for optimal transduction of retinal bipolar cells. Four-week-old rd1 mice were injected subretinal with AAV serotypes 1, 2, 5, 7, 8, and 9 carrying green fluorescent protein (GFP). Rd1 homozygous mice carry the rd1 mutation, and rod photoreceptor degeneration in these mice begins around postnatal (P) 10 days and is nearly complete by P21 days. GFP was placed under the control of the strong non-cell type specific promoter CBA (CMV immediate early enhancer fused to bovine β-actin promoter plus intron 1-exon 1 junction). After 1 month, the mice were tested for GFP expression. Double labeling with PKCα antibody from mice injected with AAV7 revealed that the transduced cells were most likely residual (no outer segment) photoreceptors rather than bipolar cells. Subretinal injections of AAV7...
Embodiment 3
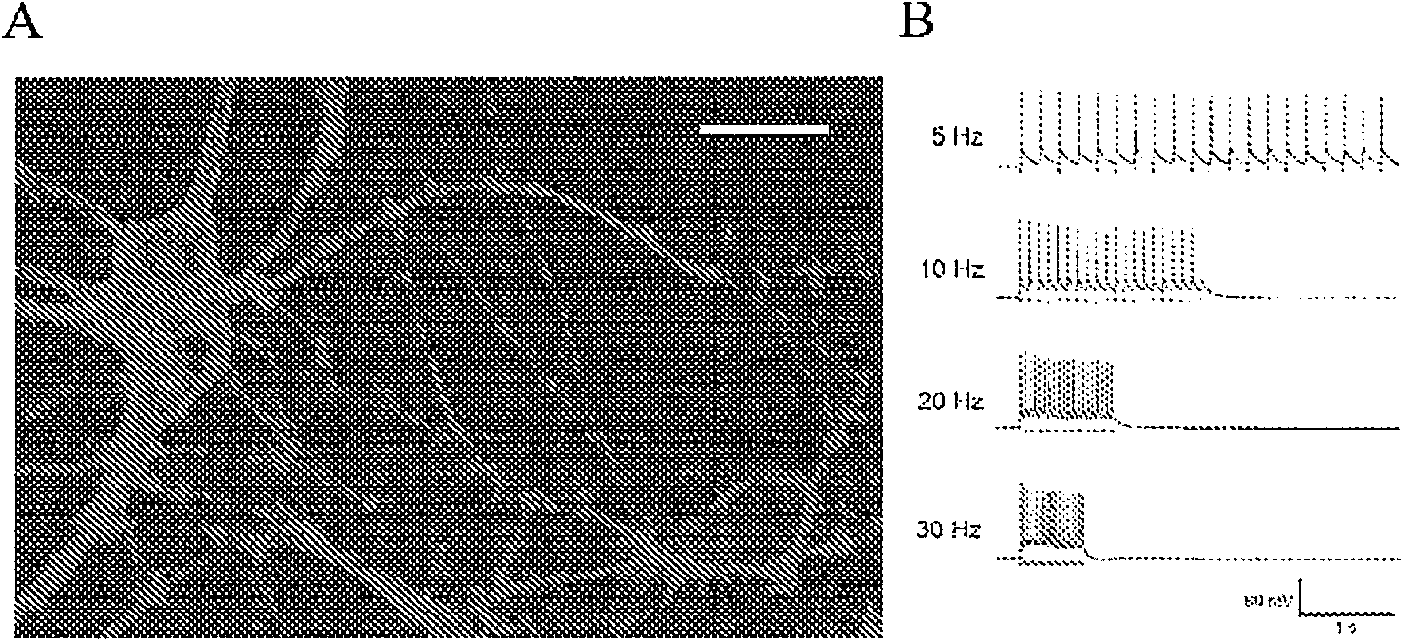
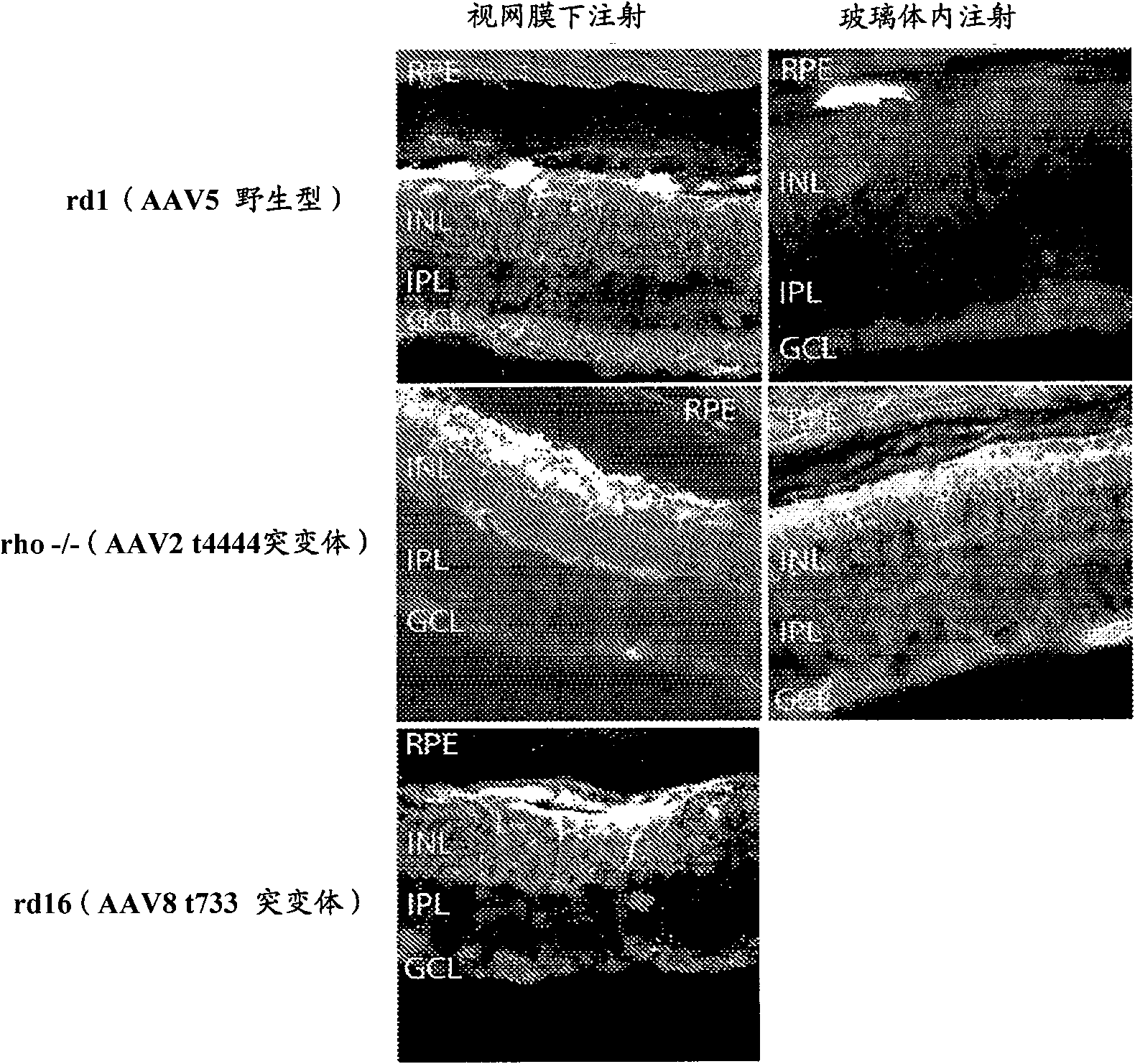
[0181] Example 3: Transduction of vision with serotype AAV5, AAV2 Y444F mutant and AAV8 Y733F mutant omental bipolar cells
[0182] Such as Figure 10 As indicated, mice were injected subretinal (right eye) and intravitreal (left eye) with 1.5 μl of different serotypes of adeno-associated virus (AAV). The serotypes tested included AAV2, AAV5 and AAV8, all of which were traditional wild-type serotypes. In addition, serotypes AAV2 Y444F mutant and AAV8 Y733F mutant with a single tyrosine to phenylalanine mutation were injected, where 444 and 733 indicate the sites of viral capsid tyrosine point mutations, respectively. The virus contains the self-complementary DNA construct GRM6-ChR2-GFP, where GRM6 is the metabotropic glutamate receptor 6 regulatory sequence driving cell-specific expression in ON bipolar cells (including rod bipolar), and ChR2 is A therapeutic light-sensitive protein gene, and GFP is a reporter gene.
[0183] Figure 10 The image in shows the overall ex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com