Osteocalcin (OC) freeze-dried powder preparation for injection
A technology of osteocalcin and freeze-dried powder, which is applied in the field of pharmaceutical preparations of osteocalcin, can solve the problem that there is no injection preparation of osteocalcin successfully developed, the stability and safety of osteocalcin cannot be guaranteed, and it is difficult to make injection preparations and other issues, to achieve good in vivo safety, easy quality control, and small batch-to-batch differences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 Preparation of lyophilized powder for injection of osteocalcin
[0022] The formula is as follows:
[0023]
[0024] The preparation method is as follows:
[0025] 1) Dosing: mix osteocalcin with auxiliary materials, add water for injection, stir to dissolve at room temperature, adjust the pH value to 8.0-10.0 with a pH regulator, and the dosage of water for injection is 4500ml;
[0026] 2) Decolorization: Add 0.03 g of activated carbon for needles to the solution prepared in step 1) according to the ratio of 0.03 g of activated carbon for needles per 100 ml of solution, stir at room temperature for 15 minutes for decolorization, filter to remove carbon, measure the pH value or further adjust the pH value , so that the pH value is within the range of 8.0-10.0, add water for injection to 6000ml, then filter with a 0.22μm microporous membrane, pack in vials, and half stopper;
[0027] 3) freeze-drying:
[0028] a. Pre-freezing: quickly freeze the solution ...
Embodiment 2
[0031] Example 2 Preparation of lyophilized powder for injection of osteocalcin
[0032] The formula is as follows:
[0033]
[0034] The preparation method is as follows:
[0035] 1) Dosing: mix osteocalcin with auxiliary materials, add water for injection, stir to dissolve at room temperature, adjust the pH value to 8.0-10.0 with a pH regulator, and the dosage of water for injection is 4500ml;
[0036] 2) Decolorization: Add 0.03 g of activated carbon for needles to the solution prepared in step 1) according to the ratio of 0.03 g of activated carbon for needles per 100 ml of solution, stir at room temperature for 20 minutes for decolorization, filter to remove carbon, measure the pH value or further adjust the pH value , so that the pH value is within the range of 8.0-10.0, add water for injection to 6000ml, then filter with a 0.22μm microporous membrane, pack in vials, and half stopper;
[0037] 3) freeze-drying:
[0038] a. Pre-freezing: quickly freeze the solution ...
Embodiment 3
[0041] Example 3 Stability investigation
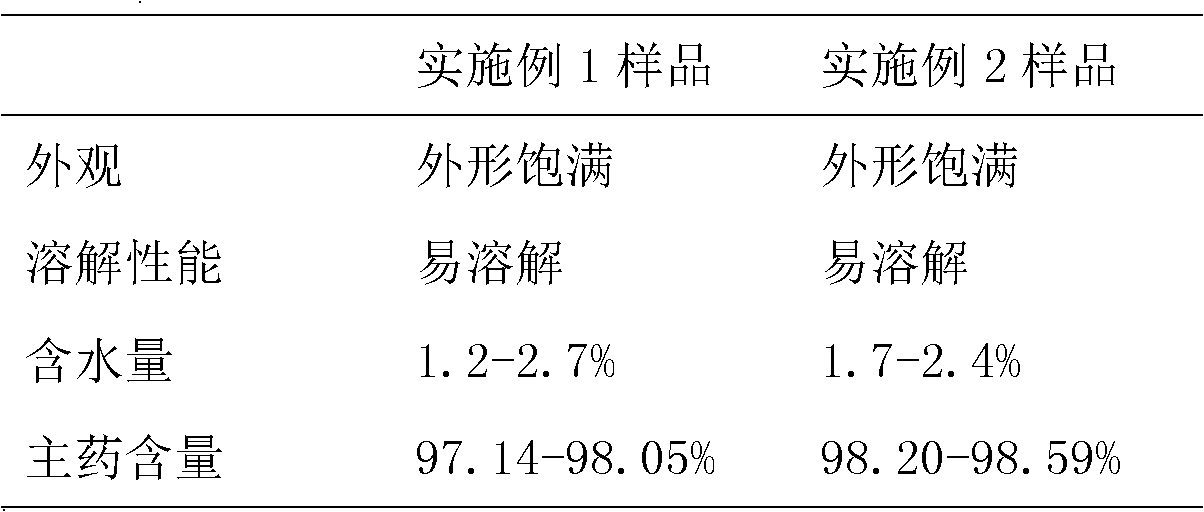
[0042] Each 4 parts of the lyophilized powder prepared by embodiment 1 and embodiment 2, 1 part is reserved at room temperature after the reference substance measures the content of the main drug, and the other 3 parts are experimental products, which are respectively placed in light (4500 ± 500) lx, high temperature (50 ±2) ℃ and high humidity (70±5)% for 180 days, samples were taken on the 60th day, 120th day and 180th day respectively, the appearance was observed, and the water content, solubility and main drug content were measured. There was no significant difference in the appearance, water content, solubility, and content of the main drug among the preparations, and no degradation or aggregation of osteocalcin occurred in the preparations. The appearance, water content, dissolubility and main drug content data of each preparation obtained by sampling during 180 days are shown in Table 1, wherein the main drug content is represe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com