Glucagons like peptide-1 (GLP-1) analog and application thereof
A technology of glucagon and GLP-1, which is applied in the field of glucagon-like peptide-1 analogs, can solve the problems of GLP-1 loss of biological activity, loss of histidine residues, etc., and achieve convenient purification work, Improved stability, easy to automate effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
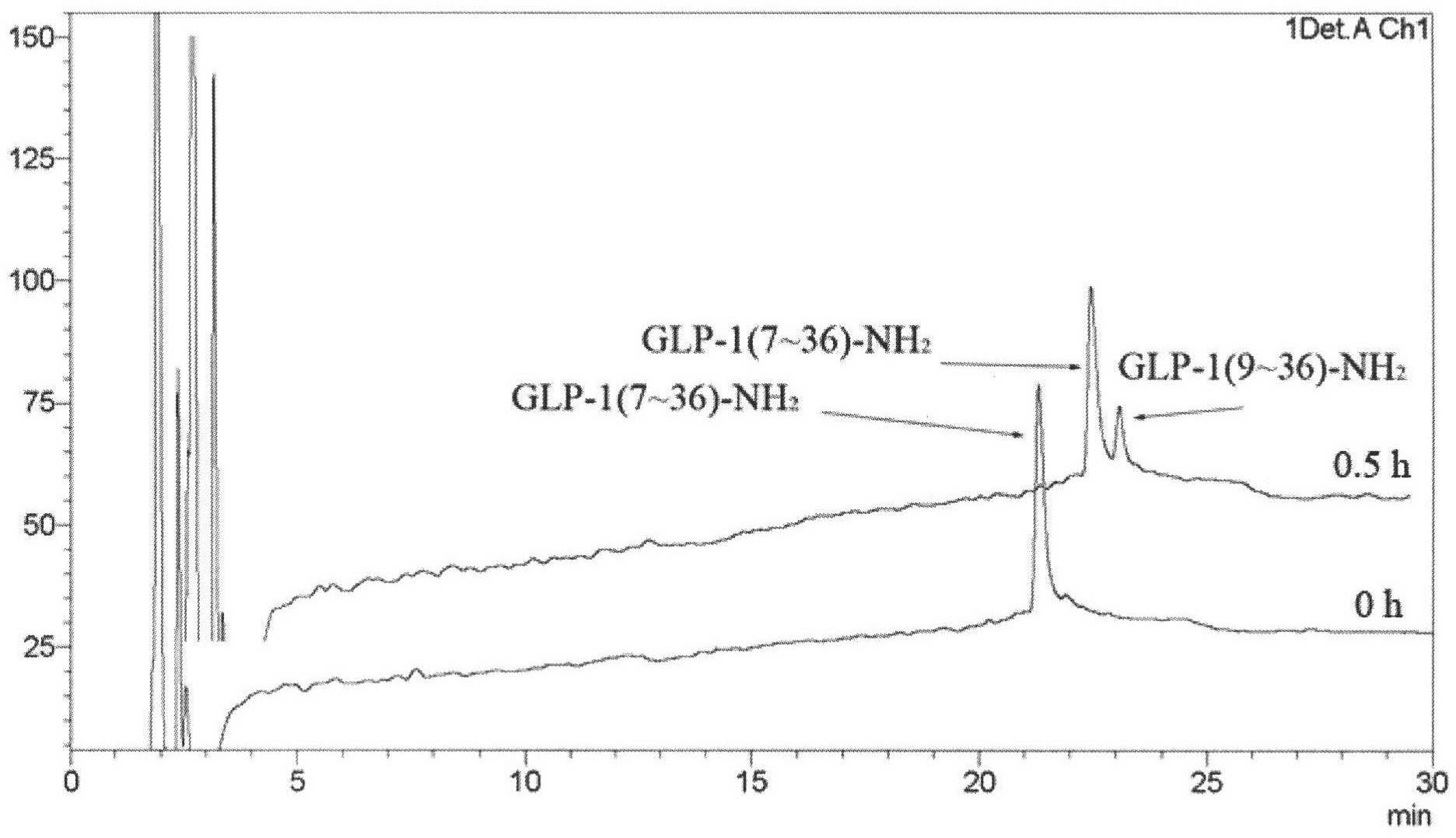
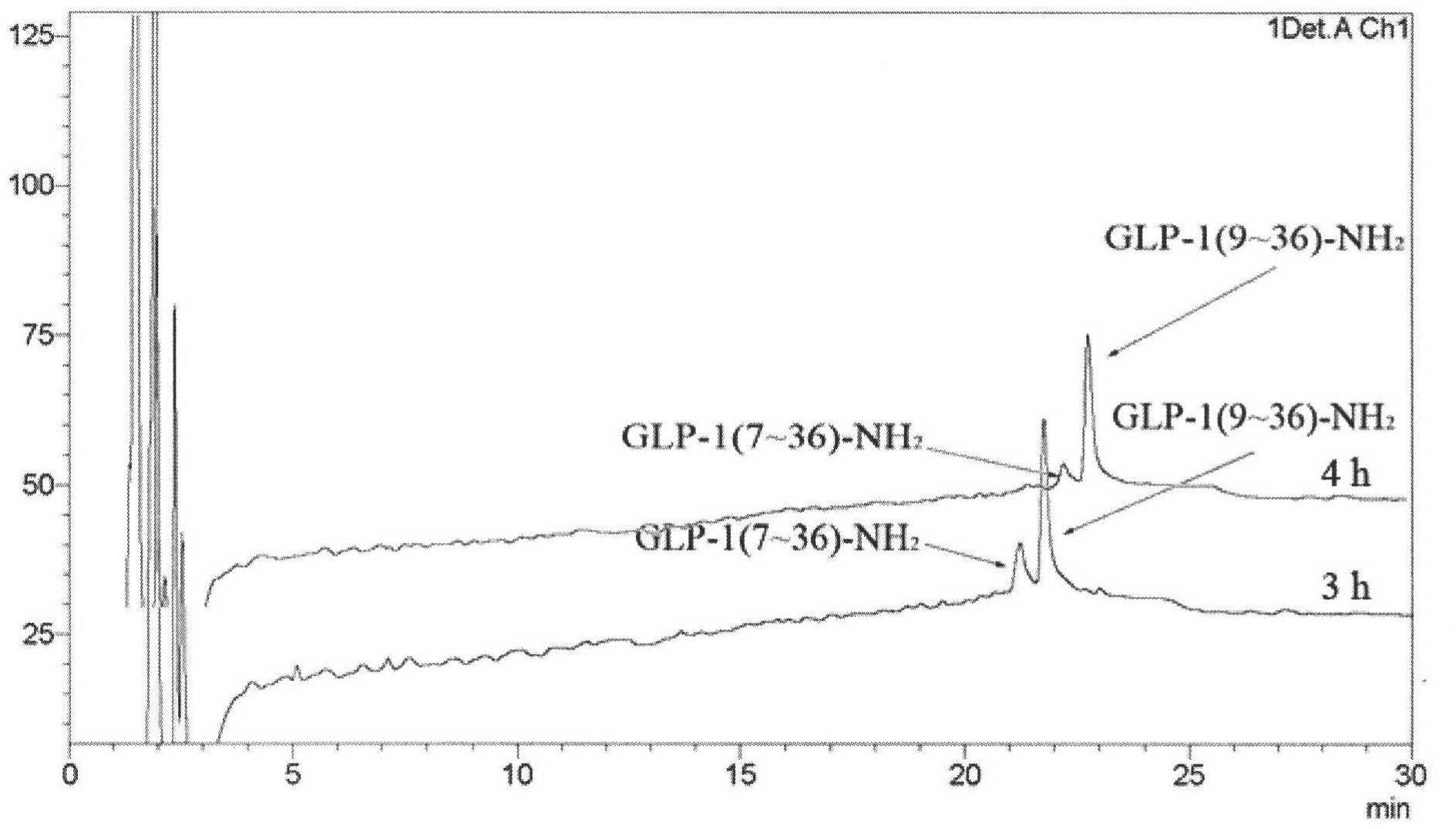
Image
Examples
Embodiment 1
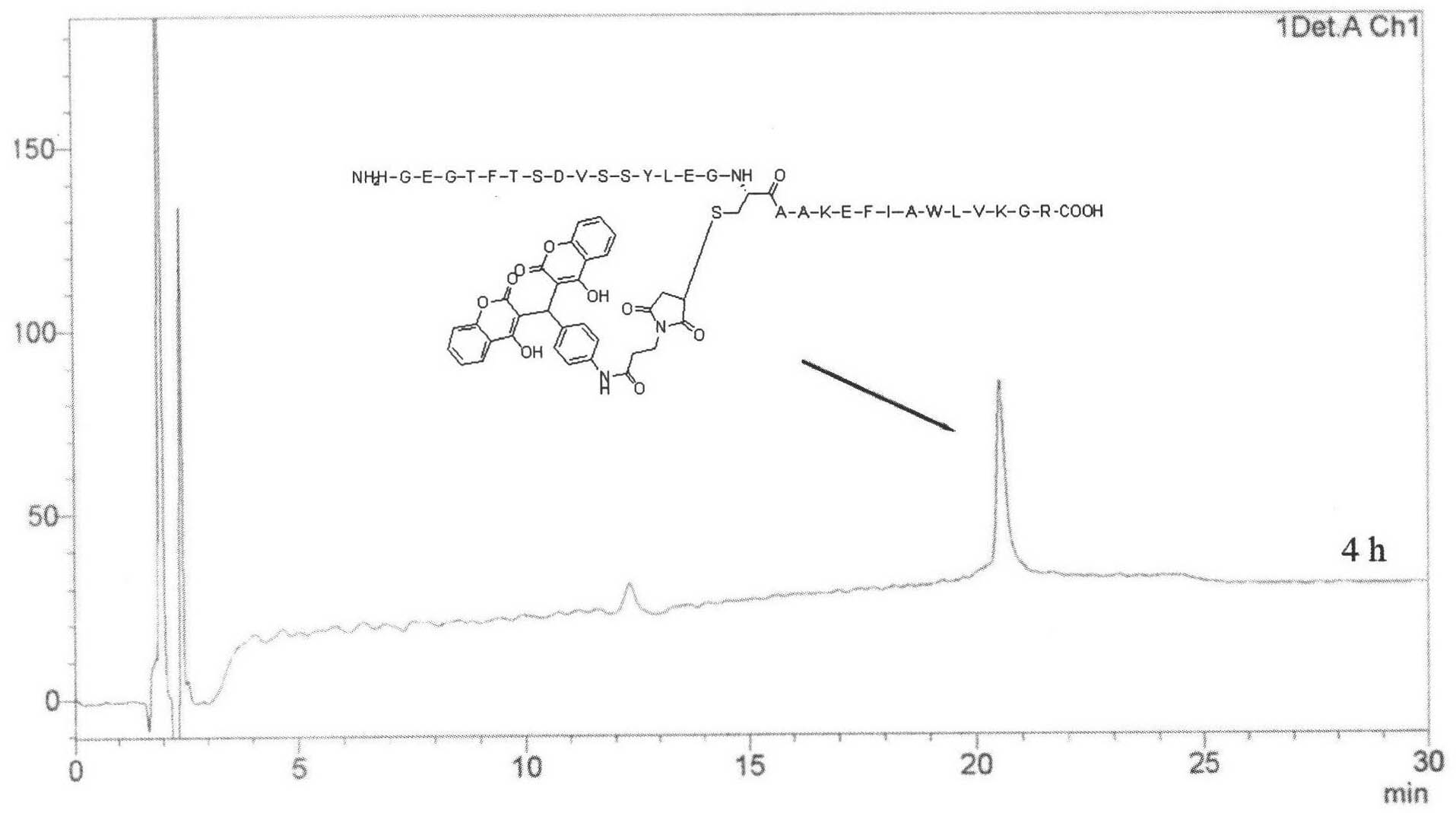
[0056] His-Gly-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Val-Ser-Ser-Tyr-Leu-Glu-Gly-Chemically Modified Cys-Ala-Ala-Lys-Glu-Phe-Ile-Ala -Trp-Leu-Val-Lys-Gly-Arg-NH 2 (SEQ. ID NO: 2)
[0057] microwave-facilitated solid-phase synthesis
[0058] (1) Swelling of the resin
[0059] Weigh 50 mg of Fmoc-Rink amide-MBHA Resin (substitution amount 0.4 mmol / g), swell with 7 mL of DCM for 30 min, filter to remove DCM, then swell with 10 mL of NMP for 30 min, and finally rinse with NMP, DCM, and 7 mL of NMP respectively.
[0060] (2) Microwave promotes removal of Fmoc protecting group
[0061] Put the swollen resin into the reactor, add 7mL of 25% piperidine / NMP (V / V) solution containing 0.1M HOBT, react in the microwave reactor for 1min, the microwave power is 15W, and the reaction temperature is controlled at 50°C Within the time period, use an air compressor to compress the air to cool, and filter the solution after the reaction; add 7 mL of 25% piperidine / NMP (V / V) solution containing 0.1M HO...
Embodiment 2~6
[0096] According to the method described in Example 1, the glucagon-like peptide-1 (GLP-1) analogues of Examples 2-6 were synthesized according to the corresponding sequence, and the respective molecular weights were confirmed by electrospray mass spectrometry (ESI-MS) .
Embodiment 2
[0098] His-Ala-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Val-Ser-Ser-Tyr-Leu-Glu-Gly-Chemically Modified Cys-Ala-Ala-Lys-Glu-Phe-Ile-Ala -Trp-Leu-Val-Lys-Gly-Arg-NH 2 (SEQ. ID NO: 3);
[0099] The theoretical relative molecular mass is 3850.8. ESI-MS m / z: found[M+4H] 4+ 963.7, [M+5H] 5+ 771.2;calu[M+4H] 4+ 963.7, [M+5H] 5+ 771.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com