Mycobacterium tuberculosis vaccine
一种结核分枝杆菌、分枝杆菌的技术,应用在抗细菌药、抗体医疗成分、细菌抗原成分等方向,能够解决免疫有限等问题,达到其他医学或兽医学应用改善、预防改善的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 - Materials and methods
[0050] Mycobacterial strains and growth conditions
[0051] M. bovis BCG was grown on Middlebrook 7H10 agar plates supplemented with oleic acid albumin dextrose catalase (OADC) (Becton Dickinson), on Dubos agar, supplemented in the presence of Tween 80 (0.05%) in liquid 7H9 medium with OADC or in Dubos broth and incubated at 37°C.
[0052] BCG zmp1 The construction of knockout mutants has been described previously (Master et al., supra). Briefly, Rv165 was cloned from BAC (Brosch et al. Infect. Immun. 66, 2221-2229 1998) contained Mycobacterium tuberculosis zmp1 (Rv0198c) and 4.4 kbp of its flanking region not I Fragment, via Nhe I digestion and removal zmp1 part (770 bp) and replaced by the kanamycin resistance cassette. The resulting suicide vector was transformed into BCG strain 1721 and selection was done on 7H10 agar supplemented with OADC in the presence of appropriate antibiotics (Sander et al., rpsL+: a domina...
Embodiment 2
[0063] Example 2 - zmp deletion increases the immunogenicity of BCG
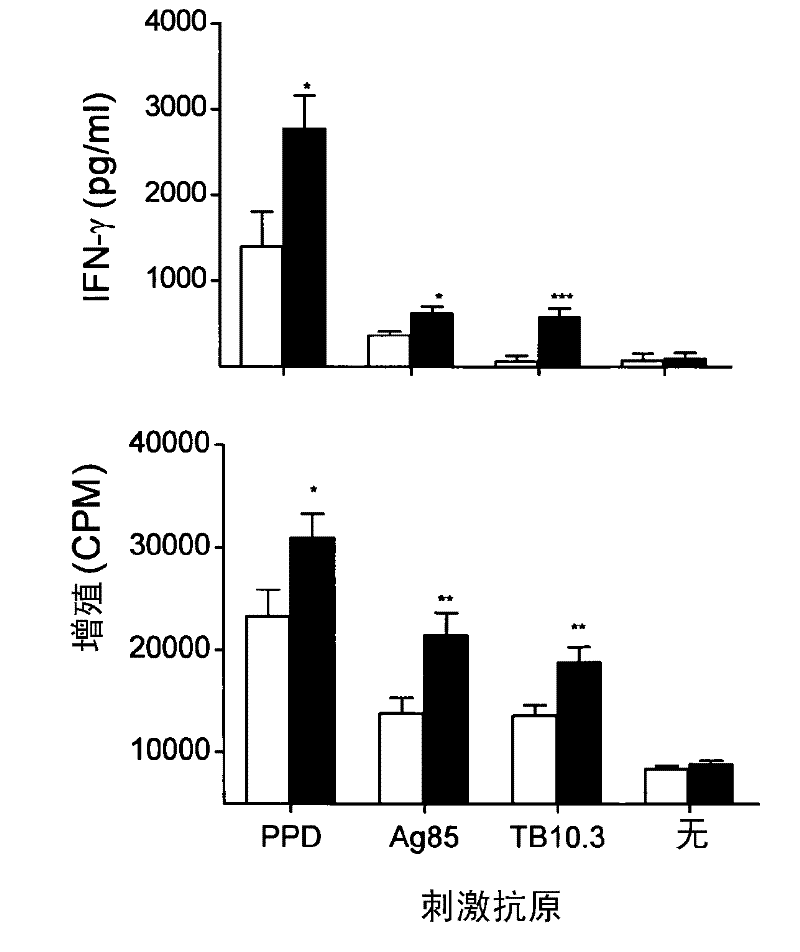
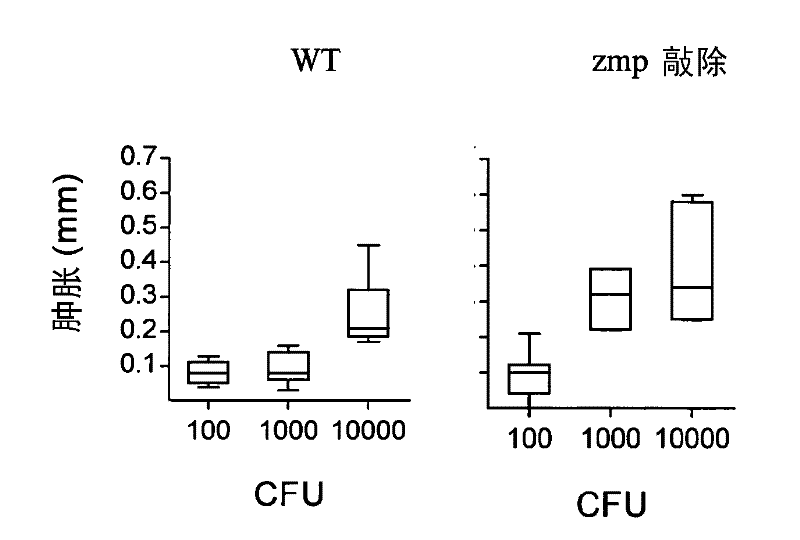
[0064] To analyze the role of Zmp1 in BCG immunogenicity, titrated doses of wild-type and zmp1 Deficient strains were used to immunize mice. DTH responses were measured after challenge with PPD footpads and different thresholds for induction were observed for the 2 vaccine strains. Although it takes 10 3 CFU inoculum of the wild-type strain produced significant footpad swelling, but with the mutant this dose one-tenth was sufficient to induce an equivalent response, at 10 4 and 10 5 Maximal footpad swelling was observed between CFUs (data not shown). We further analyze the 10 2 -10 4 Degree of footpad swelling upon inoculation. Such as figure 1 shown in the 10 3 (P=0.0006; Mann Whitney) and 10 4 (P=0.0262) CFU, zmp1 The mutant induced a significantly stronger DTH response than the BCG wild type. Experiments were repeated, and univariate ANOVA of the combined data showed no dependence on inoc...
Embodiment 3
[0067] Example 3 - zmp1 deletion increases the protective efficacy of BCG
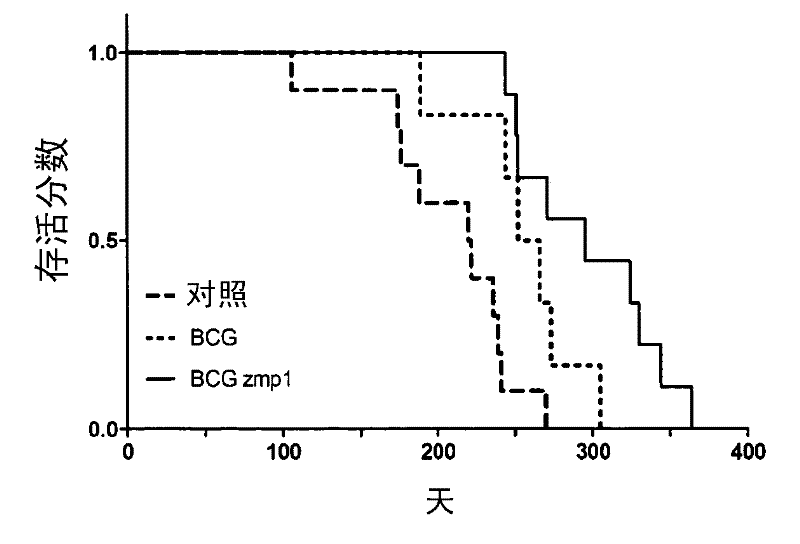
[0068] Testing Mycobacterium bovis BCG in a mouse model of tuberculosis zmp1 Protective efficacy of mutants. Mouse (C57BL / 6) group passed BCG, BCG zmp1 Mutants were immunized by subcutaneous injection, or left untreated. After 6 weeks, a low dose (10 2 CFU) M. tuberculosis H37Rv challenged mice and monitored CFU, lung pathology and mortality. Three weeks after aerosol challenge, the lungs of control mice contained high numbers of M. tuberculosis. Vaccination reduced the bacterial load in the lungs and spleen by approximately 1-2 logs in the 3 weeks prior to infection. Mice were able to control the infection and CFU counts stabilized between 3 and 23 weeks post-challenge.
[0069] 22 weeks post-infection, from all 3 groups of animals (unvaccinated control, BCG vaccinated, BCG zmp1 Lung sections from vaccinated) showed areas of lung parenchyma with reduced open alveolar spaces, variable wideni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com