Method for identifying allergenic proteins and peptides
An allergenic, protein technology, applied in the field of identification of allergenic proteins and peptides, which can solve problems such as lower-level structure homology and functional properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Example 1: Identification of cDNA sequences encoding IgE-reactive milk components and fragments thereof
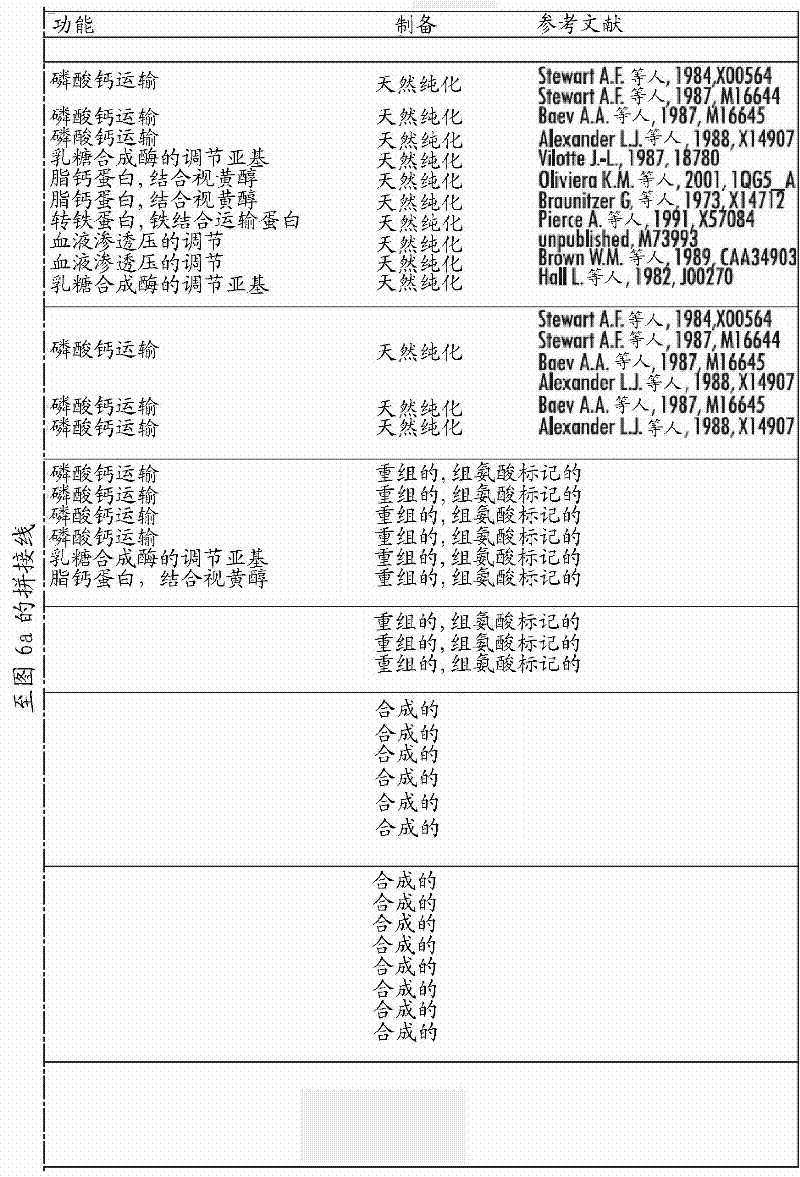
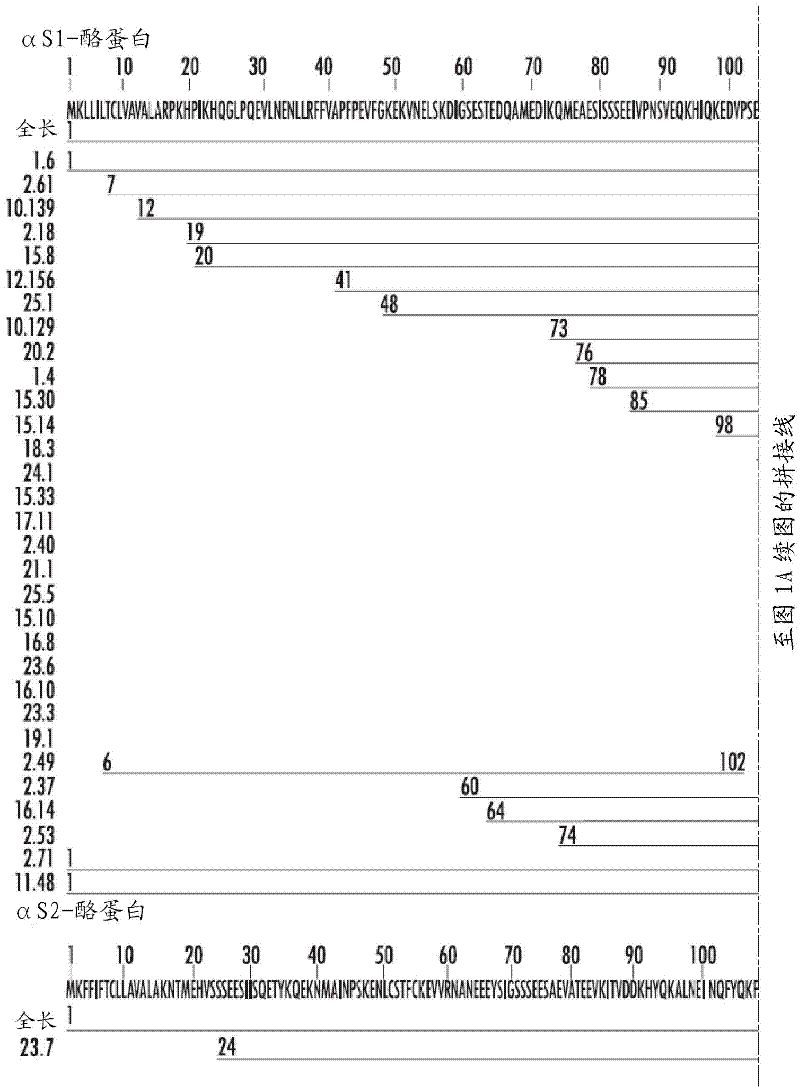
[0085] In order to identify IgE-reactive proteins and IgE-reactive protein fragments contained in milk, an expression cDNA library was first constructed using mammary gland tissue of lactating cows. The library was screened with sera from cow's milk allergic patients. cDNAs encoding IgE-reactive full-length αS1-, αS2-, β-, κ-casein and β-lactoglobulin and their IgE-reactive fragments were identified. The results were rather surprising, mammalian proteins produced in bacterial systems did not add eukaryotic post-translational modifications that sometimes play a role in IgE binding, as reported in major house dust mite allergens (Jacquet et al. , 2002). The deduced amino acid sequence is shown in Figure 1A -C (SEQ ID No. 22-84). From the resulting IgE reactive allergen fragments conclusions can be drawn about the position of the IgE epitopes.
[0086] Experimenta...
Embodiment 2
[0089] Example 2: Differences in allergenic activity and IgE reactivity of milk components
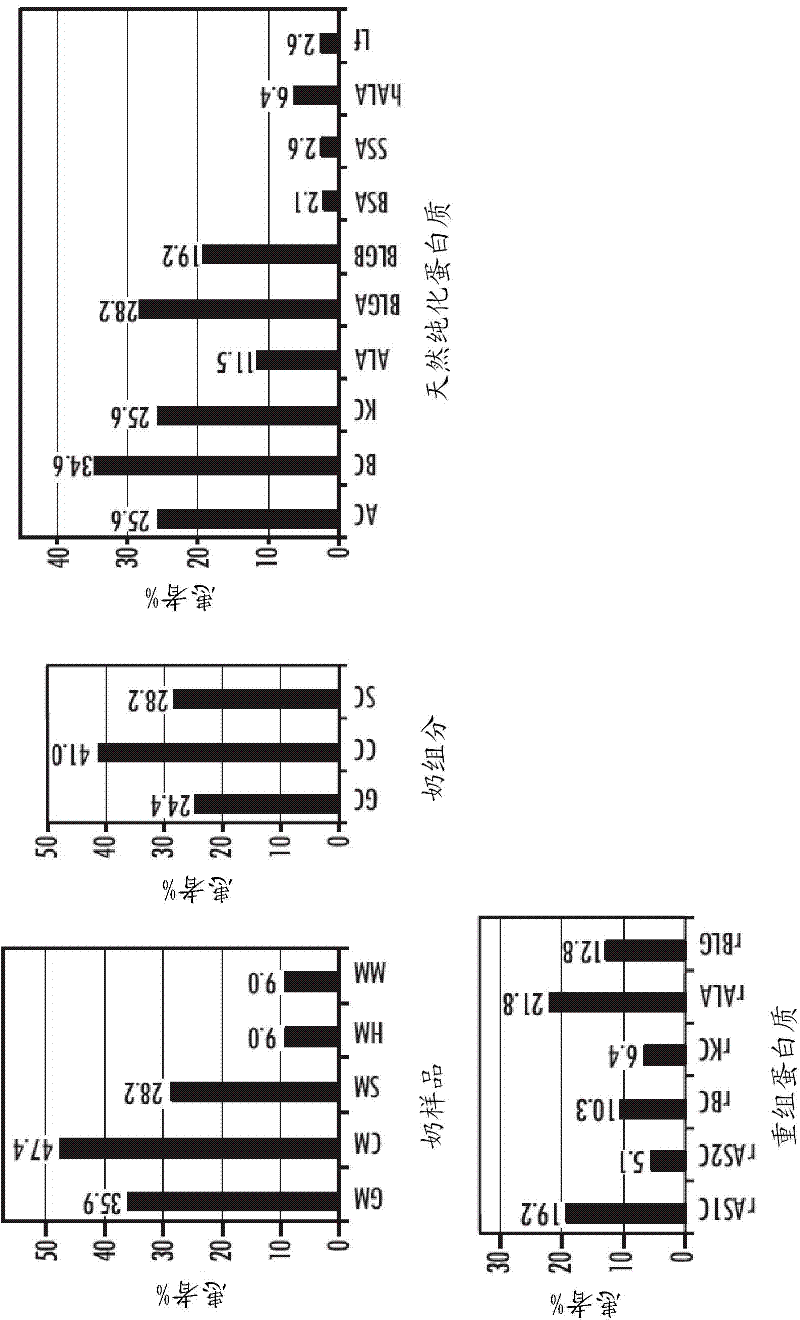
[0090] In order to assess whether a molecule has allergenic potential, not only its IgE reactivity needs to be demonstrated, but also its biological activity. A scientifically accepted model for examining molecular biological activity is the induction of histamine release from human basophils (Purohit 2005). For this, a rat basophilic leukemia cell line expressing the α-chain of the human FcεRI receptor was used (Hoffmann et al., Int Arch Allergy Immunol, Vol 126(4), p. 277-285). Different milk components including whole milk extracts of different species, milk fractions, purified native and recombinant milk proteins and recombinant protein fragments were examined (n=33). The percentage of serum IgE antibodies triggering the release of β-hexosaminidase from humanized RBL cells in response to each milk component in 78 patients was determined ( image 3 ).
[0091] The most effective ...
Embodiment 3
[0102] Example 3: Identification of proteins and / or peptides with and without allergenic activity in milk samples and milk-derived products by mass spectrometry
[0103] The sequence database of allergenic milk proteins and peptides established by the combination of IgE reactivity and allergenic activity can be used as a basis for establishing reliable and reliable methods for assessing and predicting the allergenicity of milk samples and milk-derived products. Reproducible analysis methods. Detection of potentially allergenic components in compound milk samples using mass spectrometry. As an example, a commercially available highly hydrolyzed hypoallergenic milk formulation was analyzed using a combination of mass spectrometry and upstream high performance liquid chromatography (HPLC). Figure 5 Chromatographic analysis is shown, and extracted mass spectra demonstrate that all proteins have been hydrolyzed into small peptides with a maximum length of 14 amino acids.
[0104...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com