Aromatic hydrocarbon alkyl transfer method for producing benzene and p-xylene
A technology for para-xylene and aromatic hydrocarbon alkylation, which is applied in the field of aromatic hydrocarbon transalkylation for producing benzene and para-xylene, and can solve the problems of large equipment investment and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
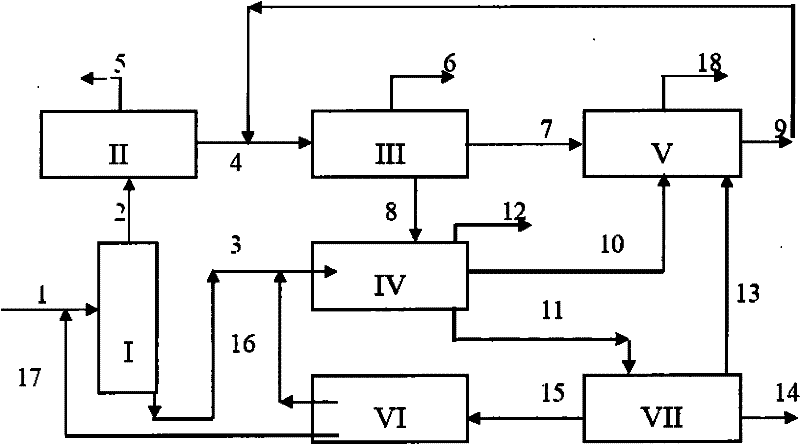
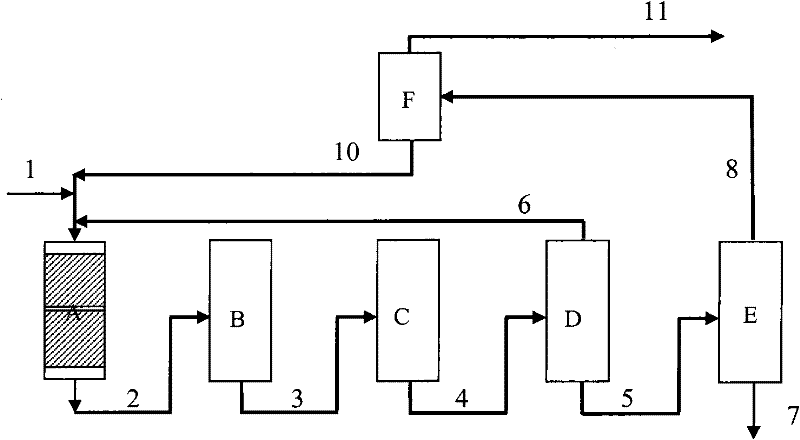
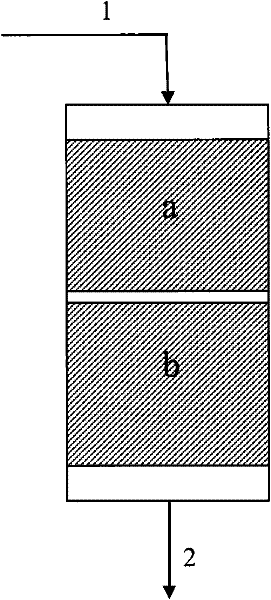
Image
Examples
Embodiment 1
[0024] Take 150 grams of high silicon-aluminum ratio hydrogen type ZSM-5 molecular sieve with a silicon-aluminum molar ratio of 180, and 70 grams of pseudo-boehmite. After kneading, extrude, dry, and roast to obtain the catalyst body, pass through an equal volume of chloroplatinic acid solution. Dipping to obtain platinum with a platinum content of 0.05% by weight, drying and roasting to obtain ethylbenzene deethylated C 8 A aromatic hydrocarbon transalkylation catalyst. Take 160 grams of low-silicon-aluminum-ratio hydrogen ZSM-5 molecular sieves with a silicon-aluminum molar ratio of 15, add 100 grams of silica sol with a silicon dioxide content of 40, knead, extrude, dry, and roast to obtain the catalyst body. The catalyst body is impregnated with base silicone oil, dried and then roasted. In this way, the silicone oil modification process is carried out twice to obtain a toluene shape-selective disproportionation catalyst.
[0025] Under the condition of hydrogen, the tolu...
Embodiment 2
[0032] Take 160 grams of hydrogen-type ZSM-5 molecular sieve with a silicon-aluminum molar ratio of 45, 80 grams of pseudo-boehmite, knead, extrude, dry, and roast to obtain the catalyst body, and obtain the platinum content by equal-volume impregnation with chloroplatinic acid solution. 0.06% by weight of platinum, after drying and roasting, ethylbenzene deethylation type C can be obtained 8 A aromatic hydrocarbon transalkylation catalyst. Take 160 grams of low-silicon-aluminum-ratio hydrogen ZSM-5 molecular sieves with a silicon-aluminum molar ratio of 25, add 100 grams of silica sol with a silicon dioxide content of 40, knead, extrude, dry, and roast to obtain the catalyst body. The catalyst body is impregnated with base silicone oil, dried and then roasted. In this way, the silicone oil modification process is carried out twice to obtain a toluene shape-selective disproportionation catalyst.
[0033] Under the condition of hydrogen, the toluene and C 8 Investigation on t...
Embodiment 3
[0039] Take 150 grams of high silicon-aluminum ratio hydrogen type ZSM-11 molecular sieve with a silicon-aluminum molar ratio of 110, and 70 grams of pseudo-boehmite, knead, extrude, dry, and roast to obtain the catalyst body, pass through an equal volume of chloroplatinic acid solution Dipping to obtain platinum with a platinum content of 0.05% by weight, drying and roasting to obtain ethylbenzene deethylated C 8 A aromatic hydrocarbon Wanyl transfer catalyst. Take 160 grams of low-silicon-aluminum-ratio hydrogen ZSM-5 molecular sieves with a silicon-aluminum molar ratio of 37, add 100 grams of silica sol with a silicon dioxide content of 40, knead, extrude, dry, and roast to obtain the catalyst body. The catalyst body is impregnated with base silicone oil, dried and then roasted. In this way, the silicone oil modification process is carried out twice to obtain a toluene shape-selective disproportionation catalyst.
[0040] Under the condition of hydrogen, the toluene and C ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com