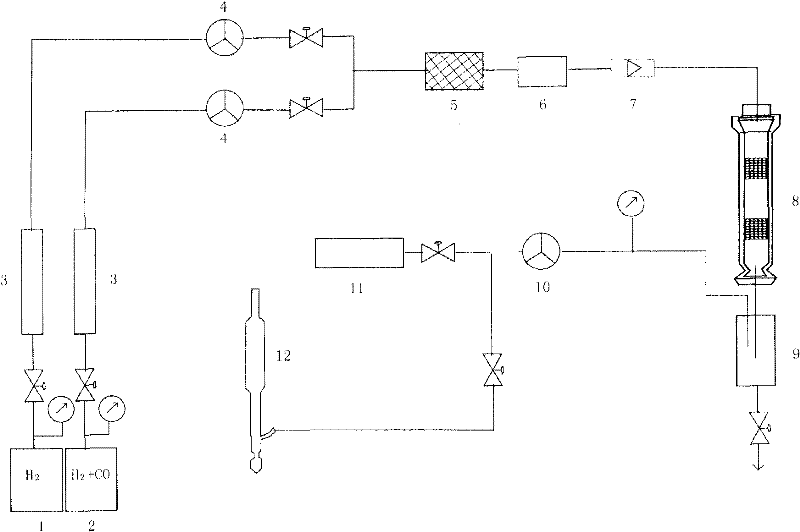
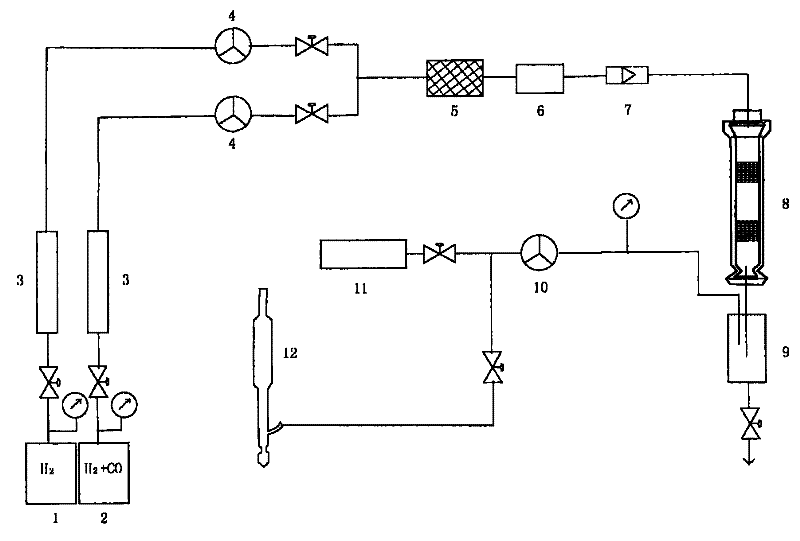
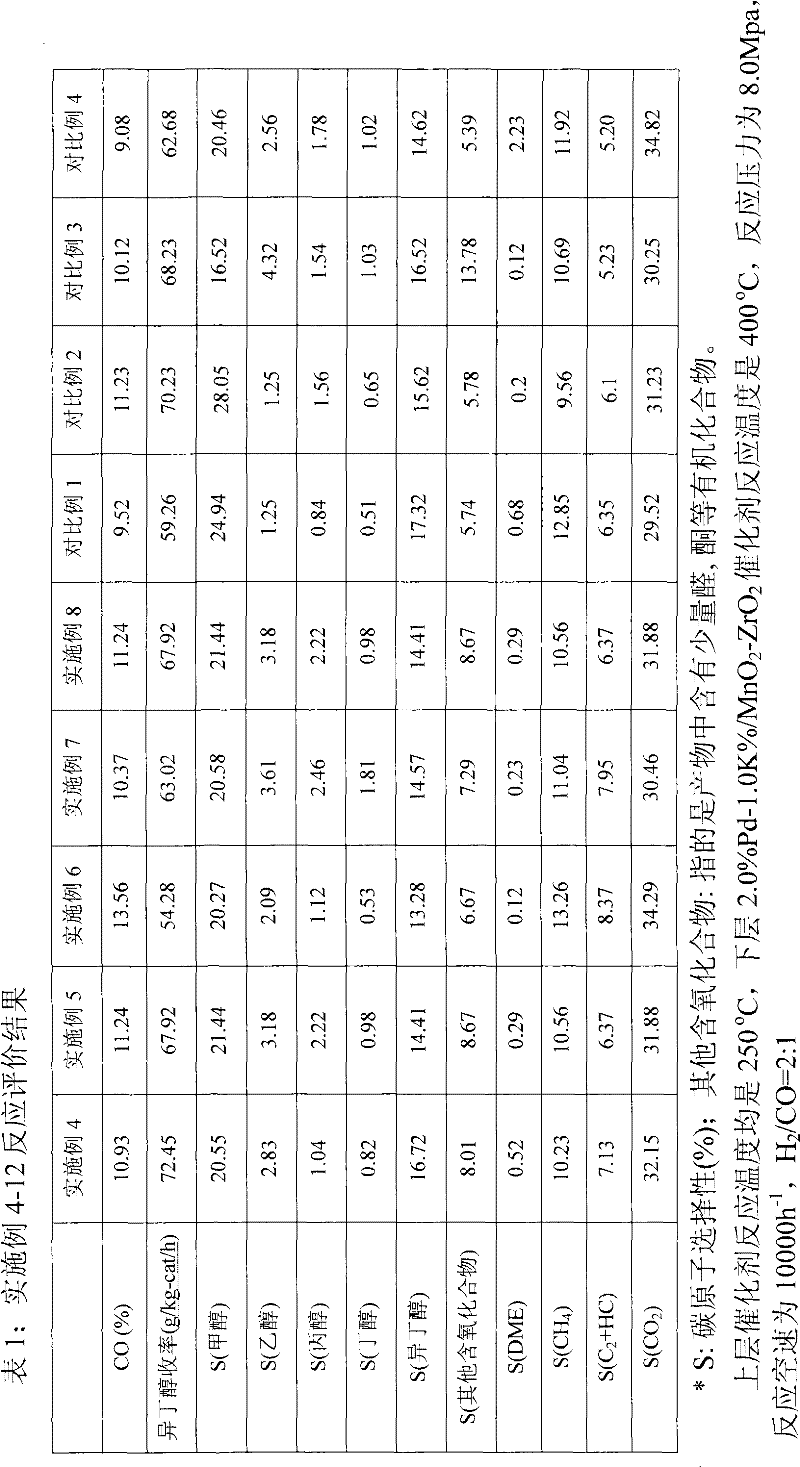
Method and device for synthesizing isobutanol through hydrogenation of carbon monoxide
A carbon monoxide and isobutanol technology, applied in chemical instruments and methods, preparation of organic compounds, preparation of hydroxyl compounds, etc., can solve the problems of complex reaction process and difficulty in industrialization, and achieve simple process, strong operability, and economical improvement sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: the preparation of Cu-Zn-Al methanol catalyst
[0040] Put 40.11g Cu(NO 3 ) 2 ·3H 2 O, 16.06g Zn(NO 3 ) 2 ·6H 2 O and 30.01gAl(NO 3 ) 3 9H 2 O was dissolved in 200ml of deionized water, and under heating and stirring, 0.5M KOH aqueous solution was co-precipitated with the above mixed solution to maintain pH=7.5-8.0. The precipitate was aged for 10 hours, and then the precipitate was filtered and washed repeatedly with deionized water until neutral. Dry at 120°C for 10 hours, sieve into 20-40 meshes, and bake at 400°C for 4 hours to obtain the prepared catalyst.
Embodiment 2
[0041] Embodiment 2: The preparation of low-carbon mixed alcohol catalyst is divided into two different catalysts.
[0042] A, the preparation of the Cu-Co-Mn catalyst modulated by alkali metal:
[0043] Put 24.88g Cu(NO 3 ) 2 ·3H 2 O, 29.97g Co(NO 3 ) 2 ·6H 2 O and 36.86g containing 50% Mn(NO 3 ) 2 The aqueous solution was dissolved in 200ml of deionized water, and under heating and stirring, 0.5M KOH aqueous solution was used to co-precipitate with the above mixed solution to maintain pH=8.5-9.0. The precipitate was aged for 10 hours, and then the precipitate was filtered and washed repeatedly with deionized water until neutral. Dry at 120°C for 10 hours, sieve into 20-40 mesh, bake at 450°C for 4 hours, and then use a load of 0.5wt% K (K 2 CO 3 as the precursor) to impregnate mixed oxides, then dry and calcinate to obtain ready-to-use catalysts.
[0044] B, the preparation of the Cu-Co-Mg catalyst modulated by alkali metal:
[0045] Put 65.96g Cu(NO 3 ) 2 ·3H ...
Embodiment 3
[0046] Example 3: Pd-K / MnO 2 -ZrO 2 preparation of
[0047] 41.51g containing 50% Mn (NO 3 ) 2 aqueous solution and 49.80g Zr(NO 3 )·5H 2 O was dissolved in 200ml of deionized water, and under heating and stirring, 0.5M KOH aqueous solution was co-precipitated with the above mixed solution to maintain pH=8.5-9.0. The precipitate was aged for 10 hours, and then the precipitate was filtered and washed repeatedly with deionized water until neutral. Dry at 120°C for 10 hours, sieve into 20-40 mesh, and bake at 600°C for 4 hours to obtain MnO 2 -ZrO 2 Mixed oxides, and then respectively with a loading of 1.0wt% K (K 2 CO 3 precursor) and a loading of 2.0wt% Pd (Pd(NO 3 ) 2 2H 2 O is the precursor) impregnating MnO 2 -ZrO 2 The mixed oxides are then dried and calcined to obtain a spare catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com