Tubercle bacillus fusion protein Mtb8.4-HspX, and preparation method and application thereof
A technology of mtb8.4-hspx and fusion protein, applied in chemical instruments and methods, bacteria, hybrid peptides, etc., can solve unconsidered and affected problems, and achieve the effect of strong cellular immune activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
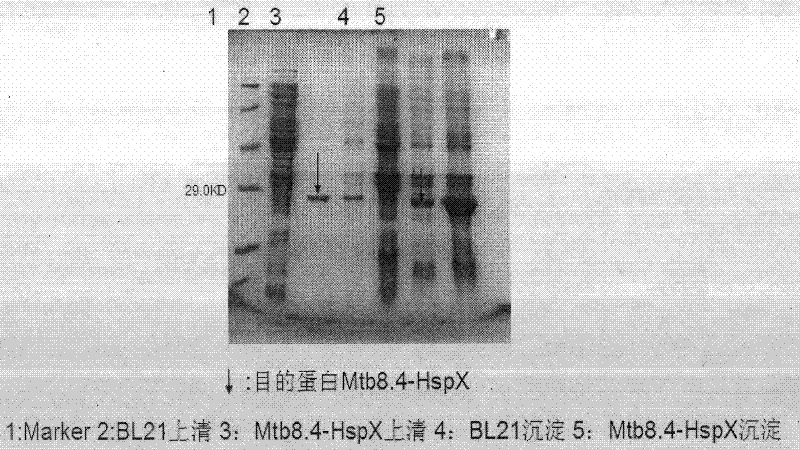
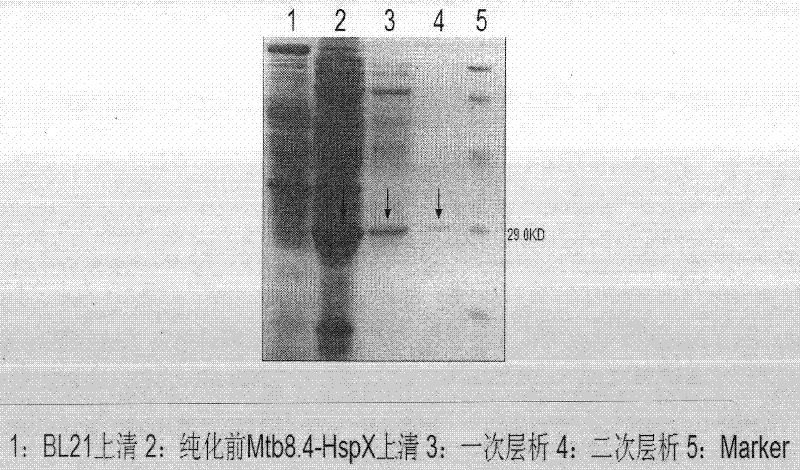
Image
Examples
Embodiment 1
[0047] 1. Construction of recombinant plasmids
[0048] The purified PCR products Mtb8.4 and Hspx and plasmid PET30a were digested with restriction endonucleases Nde I and Sac I respectively, and the digested Mtb8.4T and Hspx gene fragments and plasmid PET30a DNA fragments were passed through T4 DNA ligase After connecting overnight at 16°C, transform into Escherichia coli strain DH5α, spread on LB plates containing 50 mg / mL kanamycin, culture overnight at 37°C, pick positive clones, extract plasmids, and perform PCR and sequencing Identification. The recombinant plasmids PET30a-Mtb8.4 and PET30a-Hspx extracted and sequenced correctly were digested with restriction endonucleases Sac I and HindIII respectively, and Hspx and Mtb8.4 were digested with Sac I and HindIII respectively. After gel recovery and purification, the target gene fragments were ligated with the recovered products of PET30a-Mtb8.4 and PET30a-Hspx vectors under the action of T4 DNA ligase at 16°C overnight to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com