Therapeutic mycobacterium tuberculosis DNA vaccine and preparation method and application thereof
A technology of Mycobacterium tuberculosis and DNA vaccine, which is applied in the field of therapeutic Mycobacterium tuberculosis DNA vaccine and its preparation, and can solve the problems of drug-resistant mutation of Mycobacterium tuberculosis and the reduction of the therapeutic effect of anti-tuberculosis drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
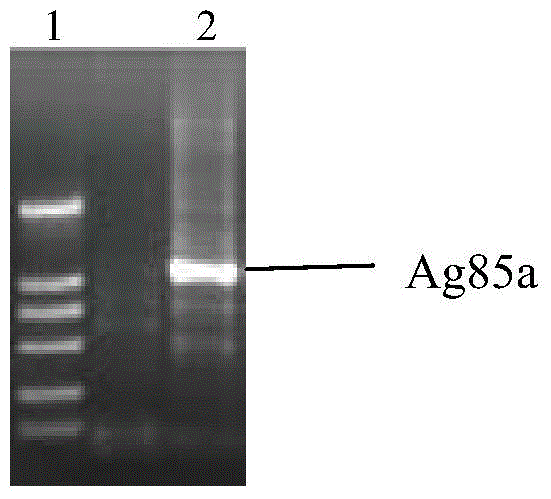
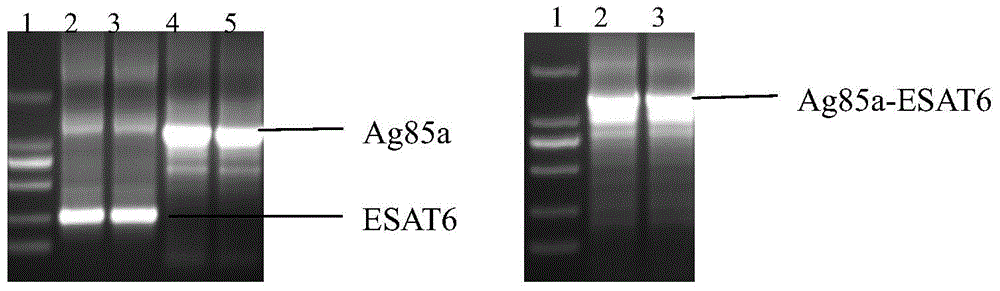
[0052] Embodiment 1 Therapeutic Mycobacterium tuberculosis DNA vaccine and its preparation method
[0053] 1. Experimental materials
[0054] 1. Strains
[0055] E.coli DH5ɑ, preserved for our laboratory.
[0056] The standard strain of Mycobacterium tuberculosis H37Rv, provided by Professor Tan Shouyong of Guangzhou Chest Hospital, has been inactivated at 121°C for 5 minutes.
[0057] 2. Eukaryotic expression vector
[0058] pVAX1 was purchased from Invitrogen.
[0059] 3. Kit
[0060] Bacterial DNA Extraction Kit (TIANamp Bacteria DNA Kit Bacterial Genomic DNA Extraction Kit, DP302).
[0061] Plasmid (miniature) extraction kit (Takara miniBEST Plasmid Purification kit ver 4.0, Code No.9760).
[0062] Gel extraction kit (Takara MinBEST Agarose Gel Extraction kit Ver3.0, D823A).
[0063] 4. Enzymes: pfu DNA polymerase (Tiangen), BamHI (Takara), XbaI (Takara), T4 DNA ligase (Takara).
[0064] 5. General reagents
[0065] Agarose, DNA Marker (Takara), Gelred TM Dye (BI...
Embodiment 2
[0142] Cell Transfection and Western Blot Detection of Example 2 Therapeutic TB DNA Vaccine
[0143] 1. Experimental materials
[0144] 1) RPMI 1640 medium (Hyclone)
[0145] 2) 10% fetal bovine serum: add 10% fetal bovine serum to the 1640 medium prepared in 1) at a volume ratio of 1:10. Fetal bovine serum was purchased from Hyclone Company.
[0146] 3) 0.25% trypsin: Asanced Technology & Industrial company.
[0147] 4) Cell line: African green monkey kidney cell COS-7, purchased from the Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences.
[0148] 8.Lipofectamine TM 2000 transfection reagent was purchased from Guangzhou Yingwei Chuangjin Biotechnology Co., Ltd.
[0149] 2. Experimental method
[0150] 1. Cell transfection experiment
[0151] 1) Inoculate cells
[0152] One day before transfection, the well-grown COS-7 cells were taken, digested with trypsin, centrifuged at 1000r / min for 5min, counted, ...
Embodiment 3
[0171] Example 3 In vivo immune test in mice of therapeutic Mycobacterium tuberculosis DNA vaccine
[0172] 1. Experimental materials
[0173] Animal immunization dose, grouping and observation time: 50 SPF-grade healthy Balb / c mice, 18-20g, 6-8 weeks old, were randomly divided into 5 groups, 10 in each group: the first group was injected with normal saline; The third to fifth groups were injected with different doses of Ag85a-ESAT6 fusion gene vaccine in the bilateral tibialis anterior muscle of mice with in vivo electric pulse, respectively 10 μg / mouse, 20 μg / mouse, 50 μg / mouse. Only. Immunization was performed every 2 weeks for a total of 3 times. The BCG group was injected only once.
[0174] The Ag85a antigen used in the experiment was purchased from Prospec Company; the ELISPOT detection kit was purchased from BD Pharmingen Company of the United States; the lymphocyte separation liquid was purchased from Thermo Company.
[0175] 2. Animal immunity
[0176] (1) Get t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com