Plant-mediated insect RNA (Ribonucleic Acid) interference reinforced by using cysteine protease
A cysteine protease and plant technology, applied in the direction of DNA / RNA fragments, plant products, plant genetic improvement, etc., can solve the problems of resurgence, decline in resistance of transgenic insect-resistant plants, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] The separation of embodiment 1 cotton cysteine protease (GhCP) and Arabidopsis cysteine protease (AtCP)
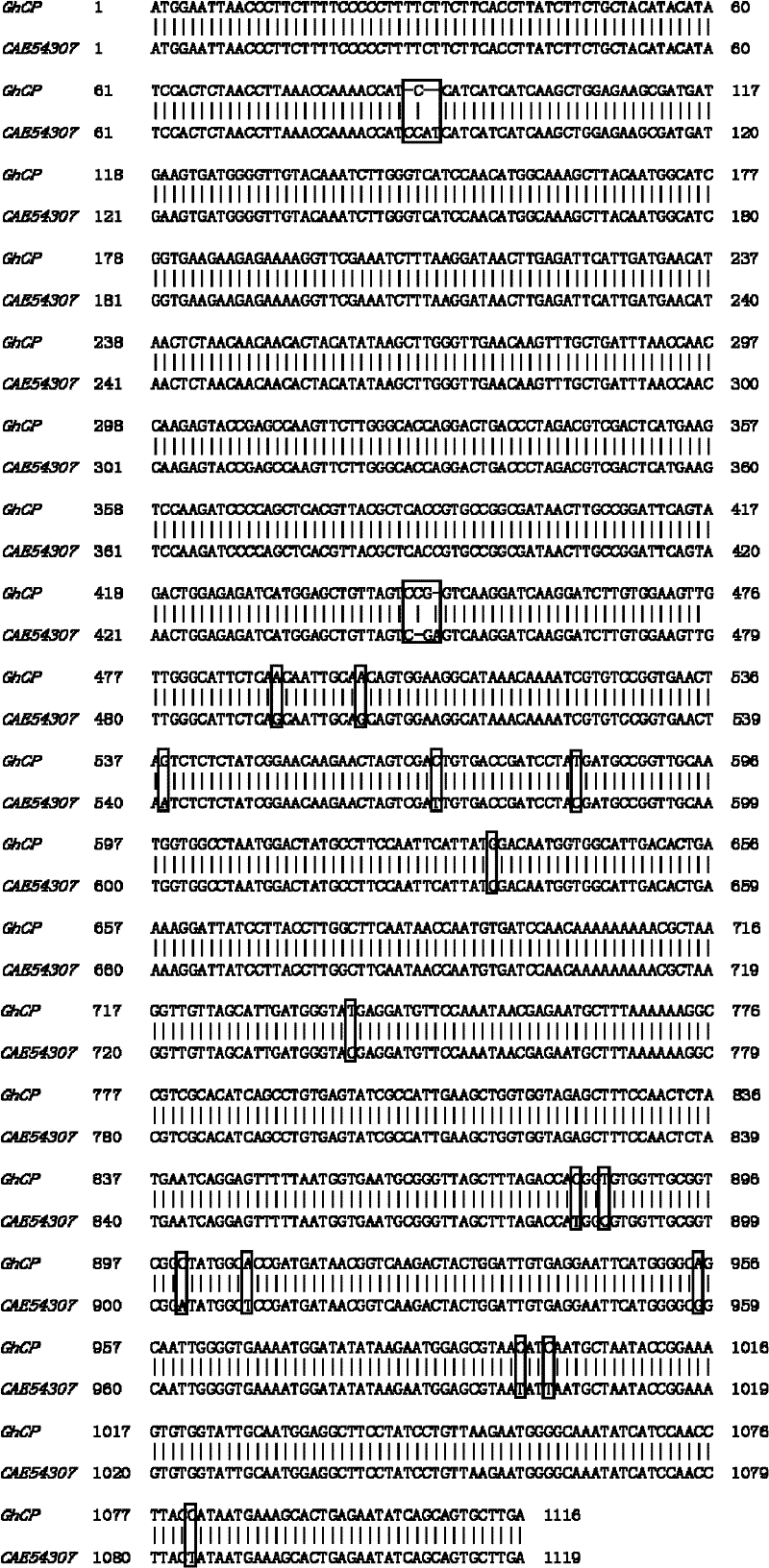
[0053] According to the sequence information of CAE54307, primers (FlGhCPF: ATGGAATTAACCCTTCTTTTC, FlGhCPR: GCAATGAATTCAAGCACTGC) were synthesized, and the gene GhCP encoding cotton cysteine protease was amplified by PCR. Sequence alignment analysis was carried out between this sequence and the known cotton cysteine protease CAE54307, and it was found that there were certain differences between GhCP and CAE54307 at the nucleotide and protein levels ( FIG. 1 ).
[0054] According to the sequence information of AT2G34080.1, primers (FlAtCPF: ATGGGTTATGCTAAATCAGC; FLAtCPR: TTAGGCAACCGAAACTTTATC) were synthesized, and the gene AtCP encoding the Arabidopsis cysteine protease was amplified by PCR. After sequence identity analysis, it was found that AtCP was consistent with AT2G34080.1.
[0055] Amino acid sequence of GhCP (SEQ ID NO: 1):
[0056] MELTLLFPLFFTLS...
Embodiment 2
[0071] Example 2 Expressing GhCP and AtCP in E.coli
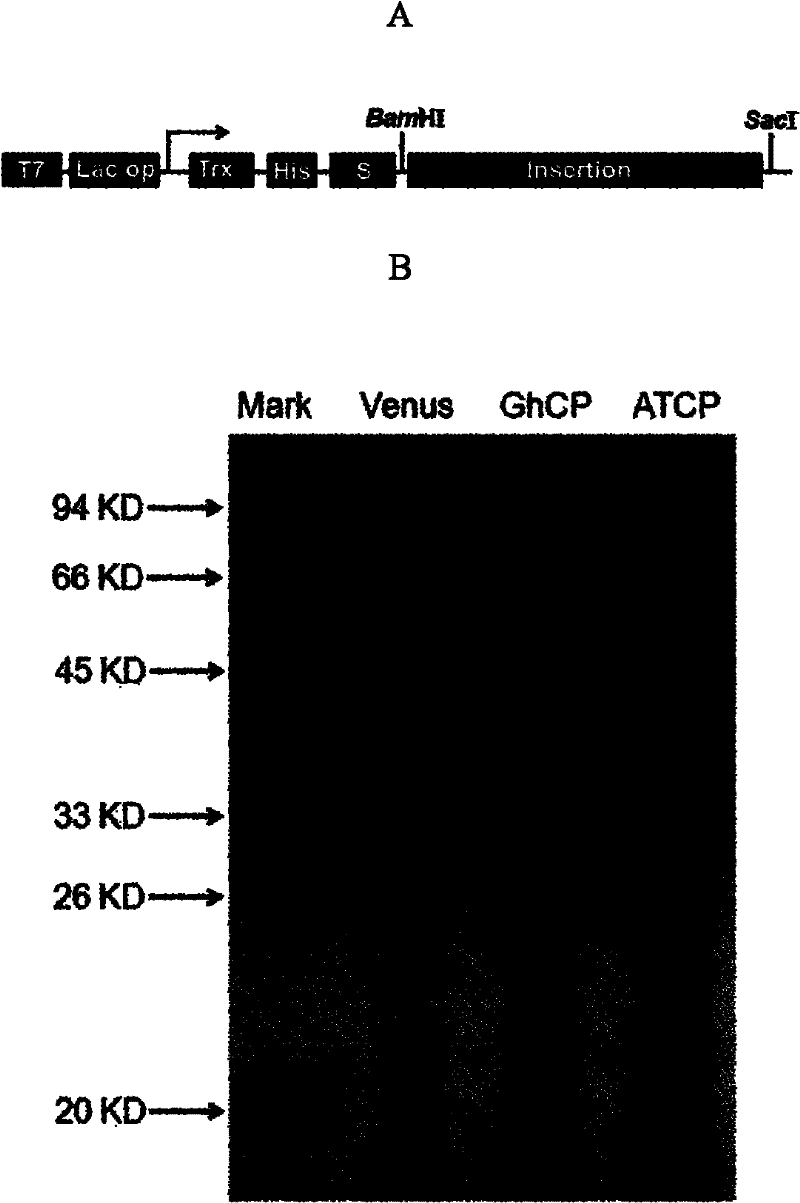
[0072] Before the start codon and stop codon of GhCP and AtCP, BamHI and SacI restriction sites were introduced respectively, and GhCP and AtCP were introduced into pET32a multiple cloning site between BamHI and SacI respectively ( figure 2 A), so as to obtain recombinant expression vectors respectively carrying corresponding target fragments, called pET32a / GhCP and pET32a / AtCP.
[0073] After transforming pET32a / GhCP and pET32a / AtCP into E.coli BL21(DE3), pick a single positive colony into 3mL LB (containing 100μg / mL Amp) medium, culture at 37°C, 220rpm until early logarithmic growth, add IPTG To a final concentration of 1 mM, continue to induce culture at 28°C for 2-3 hours. Take 1 mL of bacterial liquid and centrifuge at 12,000 g for 5 minutes, suspend the pellet in 100 μL of PBS, add an equal volume of 2×SDS loading buffer, mix and place in a boiling water bath for 5 minutes, centrifuge at 12,000 g for 10 minutes, tak...
Embodiment 3
[0075] Example 3 Cotton bollworm (Helicoverpa armigera) has increased permeability to gossypol after eating E.coli cells expressing GhCP or AtCP protein
[0076] Select the 3rd instar cotton bollworms with consistent growth and divide them into two groups, feed respectively the artificial diet mixed with the E.coli cells expressing Venus protein or GhCP (get 250ml OD as the bacterial liquid of 1.0, centrifuge, get the sediment, and 25g artificial diet (artificial For the formula and feeding method of the feed, see "Handbook of Insect Artificial Feed" such as Wang Yannian) mixed). After eating for two days, they were transferred to artificial feed containing 0.1% (mg / g) gossypol concentration, and continued to cultivate for one day. Stained with phloroglucinol to detect the content of gossypol in midgut cells, and found that the content of gossypol in midgut cells was significantly higher in larvae pretreated with E.coli cells expressing GhCP than in E.coli cells expressing Ven...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com