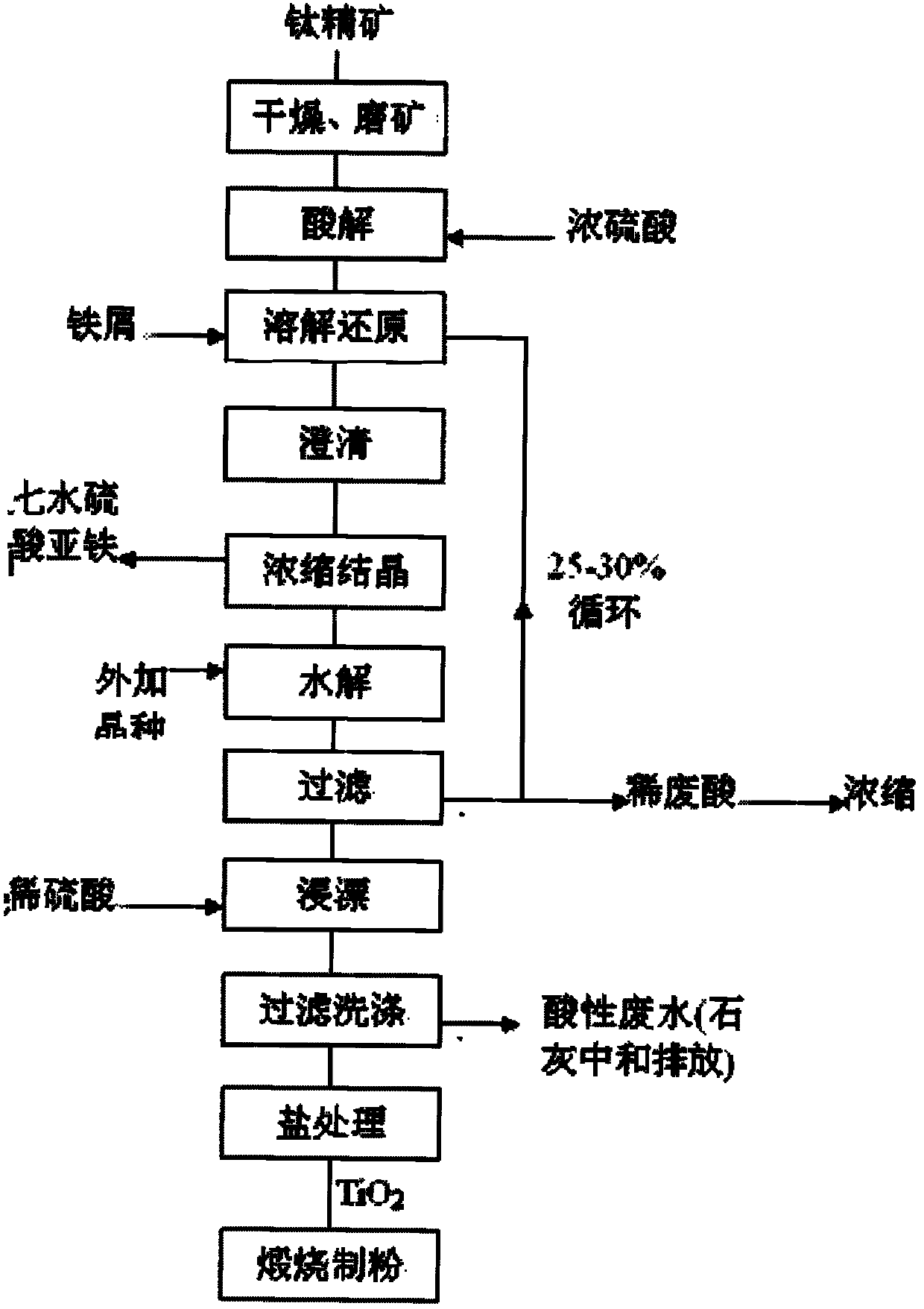
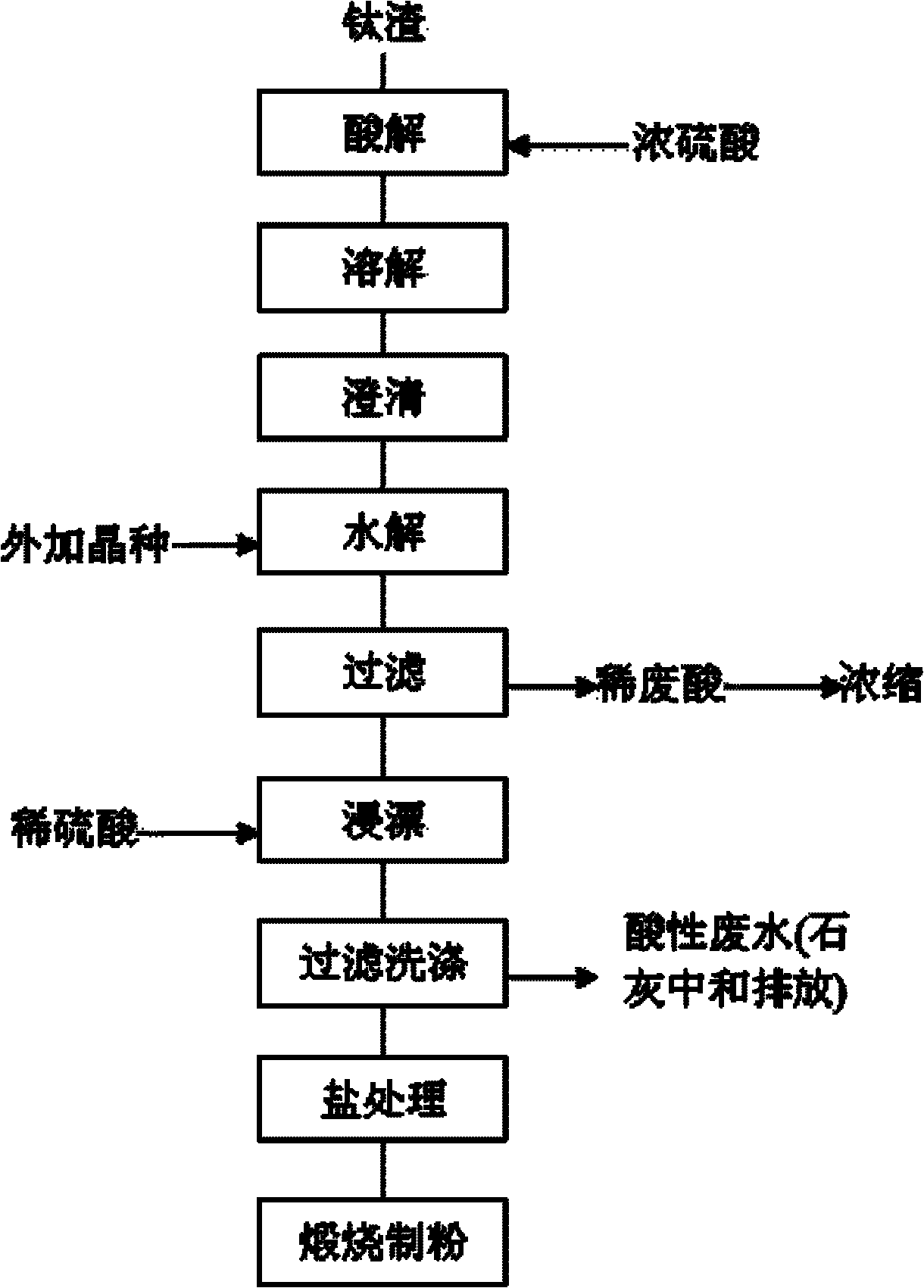
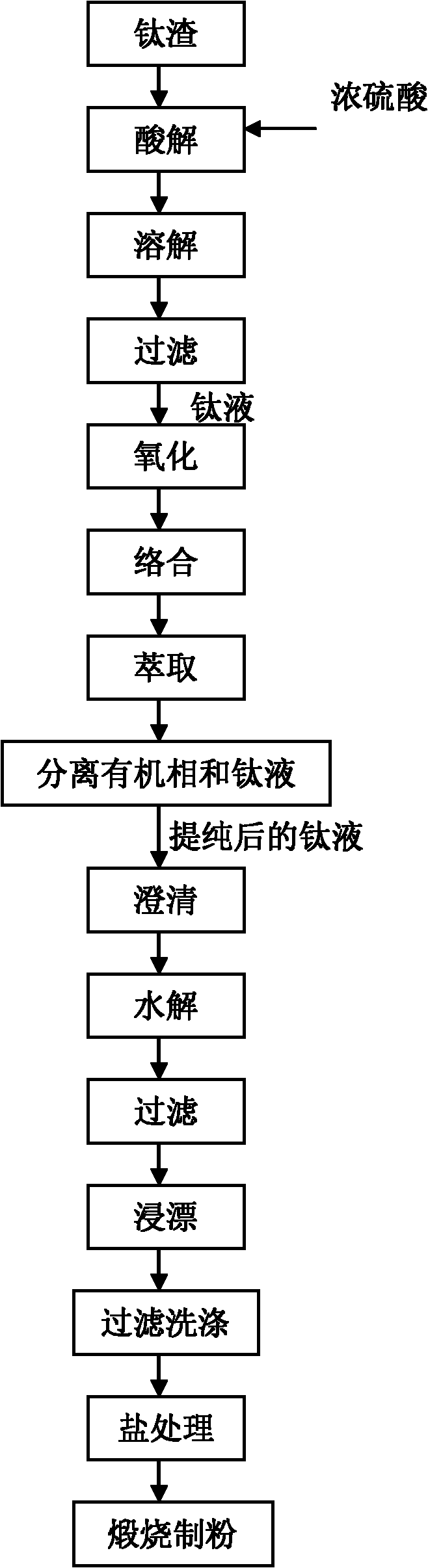
Method for purifying titanium solution
A purification method and technology of titanium liquid, which is applied in the field of titanium liquid purification, can solve the problems of little waste acid, waste water research, etc., and achieve the effect of reducing the number of water washing stages, the number of washing times, and the reduction of production and discharge
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] The preparation of embodiment 1 high titanium hydrochloric acid leaching residue
[0054] According to the hydrochloric acid direct leaching method disclosed in Chinese patent application 200910306494.5, high titanium hydrochloric acid leaching residue is prepared, and the process parameters are as follows:
[0055] A, mixing ilmenite and hydrochloric acid, the ratio of ilmenite quality (grams) to the volume (ml) of hydrochloric acid solution is 1: 2.5~5, the concentration of hydrochloric acid is 18%~30%, heating to 70 ℃~150 ℃ for leaching Reaction, the reaction time is 1-8 hours;
[0056] B. Filtrate the reaction solution and dry the obtained solid, which is the acid-soluble pertitanium hydrochloric acid leaching residue; when drying in step B, control the temperature below 350° C., and the drying time is 4-8 hours.
[0057] Detailed raw material proportioning and reaction conditions are as follows in the present embodiment:
[0058] 1. Measure 45 liters of 20% hydro...
Embodiment 2
[0067] The preparation of embodiment 2 titanium liquid
[0068] The sulfuric acid method is used to prepare titanium solution by using high titanium hydrochloric acid leaching slag as raw material, which can be carried out according to the method disclosed in Chinese patent application 200910306494.5. Specifically, the process parameters in the present embodiment are as follows:
[0069] with 72% TiO 2 20Kg of the high titanium hydrochloric acid leaching residue (prepared by the method of Example 1), 19.4 liters of 98% concentrated sulfuric acid was added, and the weight ratio of the high titanium hydrochloric acid leaching residue to 100% concentrated sulfuric acid was 1: 1.75. Stir well and start heating. Heating to about 175°C with stirring allowed the reaction to proceed spontaneously. After continuing to stir for about 5 minutes, the reactant formed a viscous solid mixture, and the mixture was aged at 180° C. and kept at the temperature for 4 hours. Cool to room tempe...
Embodiment 3
[0072] The purification of embodiment 3 titanium liquid
[0073] Prepare titanium solution according to Example 2, take 2 liters of titanium solution, and its chemical composition is shown in Table 3. First, 1.5 equivalents (relative to ferrous ions) of chlorine gas is introduced into the titanium solution to oxidize ferric ions into ferric ions. After being oxidized by chlorine gas, the chemical composition of the titanium solution is shown in Table 4.
[0074] Chemical composition of titanium liquid after table 4 oxidation
[0075]
[0076] In the oxidized titanium solution, add 360 milliliters of 11 molar hydrochloric acid to complex ferric ions. After adding hydrochloric acid, the chemical composition of the titanium solution is shown in Table 5.
[0077] The chemical composition of titanium liquid after adding hydrochloric acid in table 5
[0078]
[0079] 2.36 liters of titanium solution added with hydrochloric acid is fully mixed and contacted with 1 liter of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com