Method for preparing 2,5-dimethyl phenol through direct catalytic hydroxylation of p-xylene
A technology of dimethyl phenol and catalyzed hydroxyl group, which is applied in the field of preparation of aromatic phenolic compounds, can solve the problems of pollution, many reaction steps, and low utilization rate of atoms, and achieve the effects of easy acquisition, few reaction steps, and recyclable solvents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
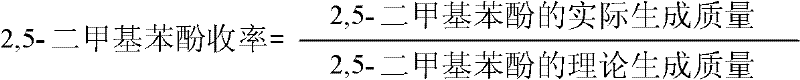
[0017] Add 16.0g acetonitrile, 0.08g PG8, 0.8g p-xylene, 18.7g 30% H 2 o 2 , reacted at 60°C for 5 hours. Samples were taken for gas chromatographic analysis. The conversion of p-xylene was 55.9%, and the yield of 2,5-dimethylphenol was 10.1%.
Embodiment 2
[0019] Add 12.0g acetonitrile, 0.04g PG8, 0.8g p-xylene, 16.0g 30% H 2 o 2 , reacted at 40°C for 4 hours. Samples were taken for gas chromatographic analysis. The conversion of p-xylene was 47.8%, and the yield of 2,5-dimethylphenol was 8.6%.
Embodiment 3
[0021] Add 24.0g of acetonitrile, 0.16g of PG8, 0.8g of p-xylene, 24g of 30% H 2 o 2 , reacted at 70°C for 7 hours. Samples were taken for gas chromatographic analysis. The conversion of p-xylene was 67.4%, and the yield of 2,5-dimethylphenol was 9.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com