4-(4-(3-trifluoro methyl) benzamido phenoxyl)-2-(methyl carbamyl) pyridine salt, its preparation method and application
A technology of benzamidophenoxy and methylcarbamoyl, applied in the field of chemical medicine, can solve the problems of low solubility, hinder the timely absorption of drugs, and low absolute bioavailability of oral administration, and achieve high solubility and bioavailability. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 Preparation of 4-(4-(3-trifluoromethyl)benzamidophenoxy)-2-(methylcarbamoyl)pyridine hydrochloride
[0046] 1. Dissolve 4-(4-(3-trifluoromethyl)benzamidophenoxy)-2-(methylcarbamoyl)pyridine in ethanol, add hydrochloric acid solution, and adjust pH=2.
[0047] 2. Filter and recrystallize the residue from ethyl acetate and water.
Embodiment 2
[0048] Example 2 Preparation of 4-(4-(3-trifluoromethyl)benzamidophenoxy)-2-(methylcarbamoyl)pyridine 4-methylbenzenesulfonate
[0049] Suspend N-methyl-4-(4-(3-trifluoromethyl)benzamido)phenoxy)pyridinecarboyl in ethyl acetate and heat to 70°C to dissolve. Within 3 minutes, a mixture of p-toluenesulfonic acid, ethyl acetate and water was added dropwise, and the reaction was heated. Cooled and filtered, rinsed the resulting solid with ethyl acetate, dried under reduced pressure to obtain 66.1g (90% yield) 4-(4-(3-trifluoromethyl)benzamidophenoxy)-2-( Methylcarbamoyl)pyridine 4-methylbenzenesulfonate crude product.
[0050] The crude 4-(4-(3-trifluoromethyl)benzamidophenoxy)-2-(methylcarbamoyl)pyridine 4-methylbenzenesulfonate was recrystallized from ethyl acetate and water to obtain Refined products, the identification data of refined products are as follows:
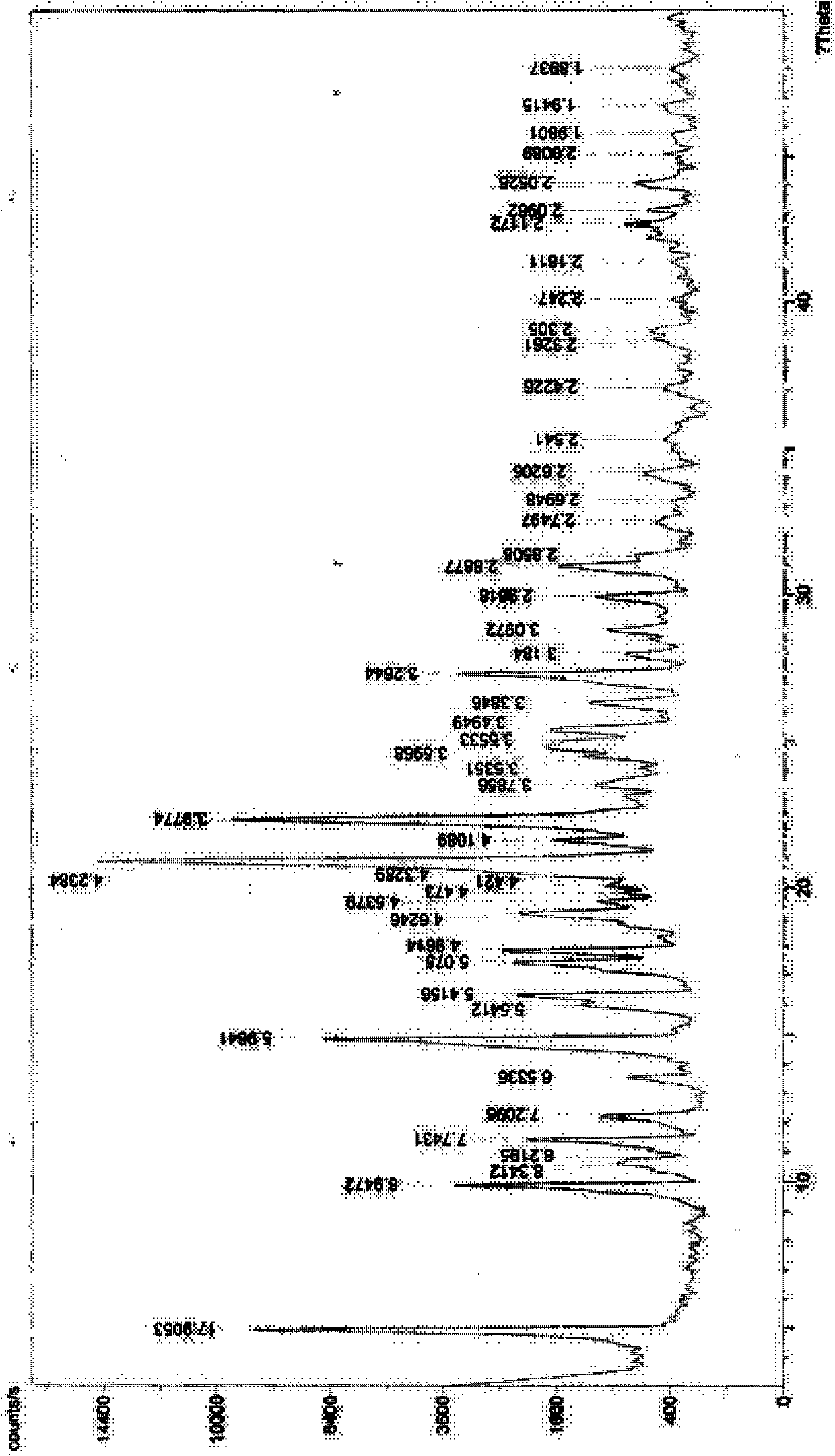
[0051] 1 H-NMR (DMSO-d 6 )δ: 2.29 (3H, s), 2.81 (3H, d, J = 4.8Hz), 6.15 (1H, s), 7.12 (2H, d, J = 8.0Hz), 7.23 ...
Embodiment 3
[0057] Example 3 Preparation of 4-(4-(3-trifluoromethyl)benzamidophenoxy)-2-(methylcarbamoyl)pyridine 4-methylbenzenesulfonic acid crystal form B
[0058] 4-(4-(3-trifluoromethyl)benzamidophenoxy)-2-(methylcarbamoyl)pyridine 4-methylbenzenesulfonate was added to a clean 2L three-necked flask, and then Add ethyl acetate into the three-necked flask. Install the condenser and thermometer. Turn on stirring and heating. When the temperature rose to 70°C, deionized water was added dropwise into the three-necked flask. After about 3 minutes, the reaction system was completely dissolved. Filtrate hot, add 200ml ethyl acetate, air cool down naturally, stir and crystallize. After 2 hours, the reaction system was filtered to obtain a white solid. The solid was rinsed with ethyl acetate and sucked dry. Drying under reduced pressure gave 59.1 g of solid product (yield 89.6%).
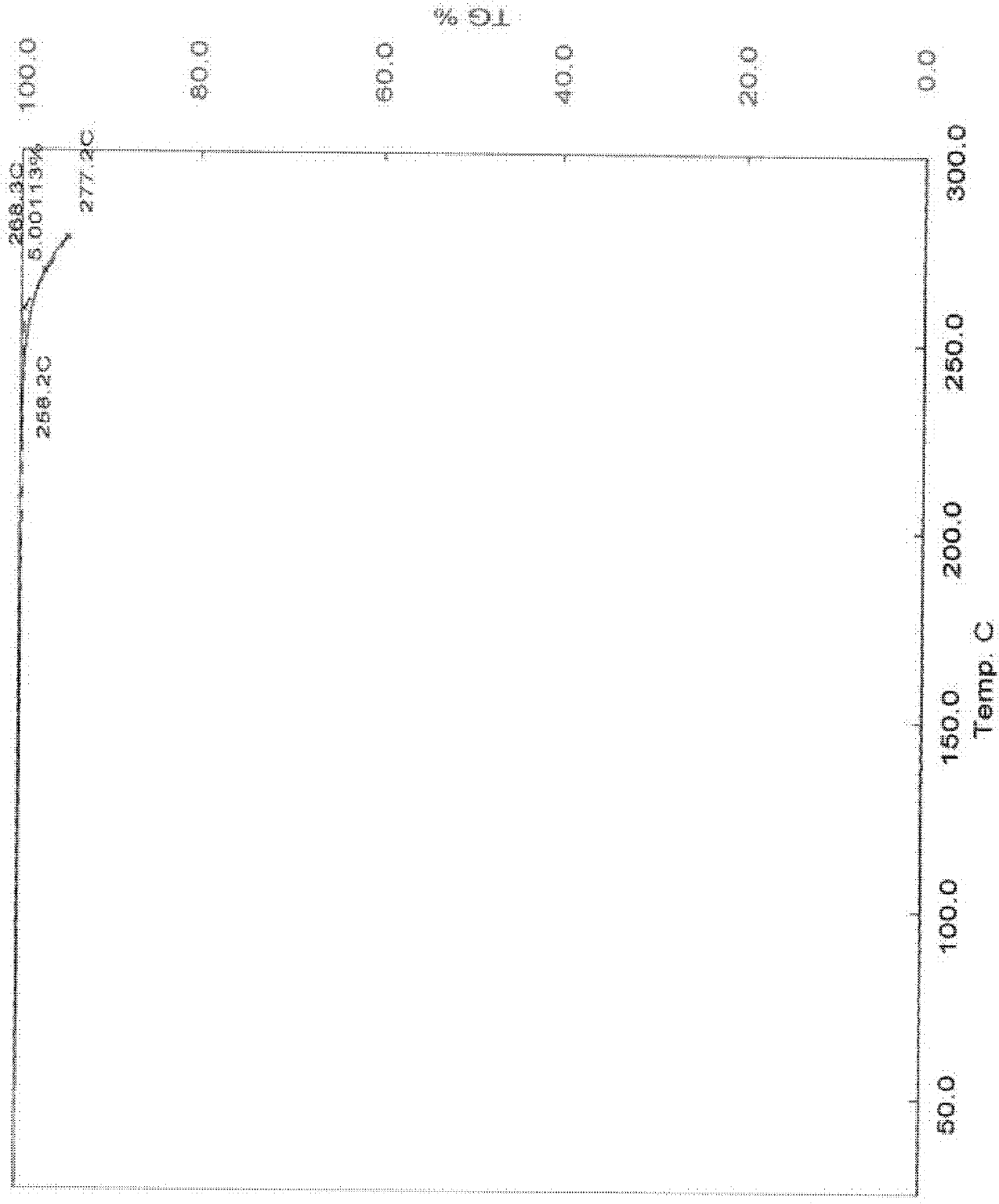
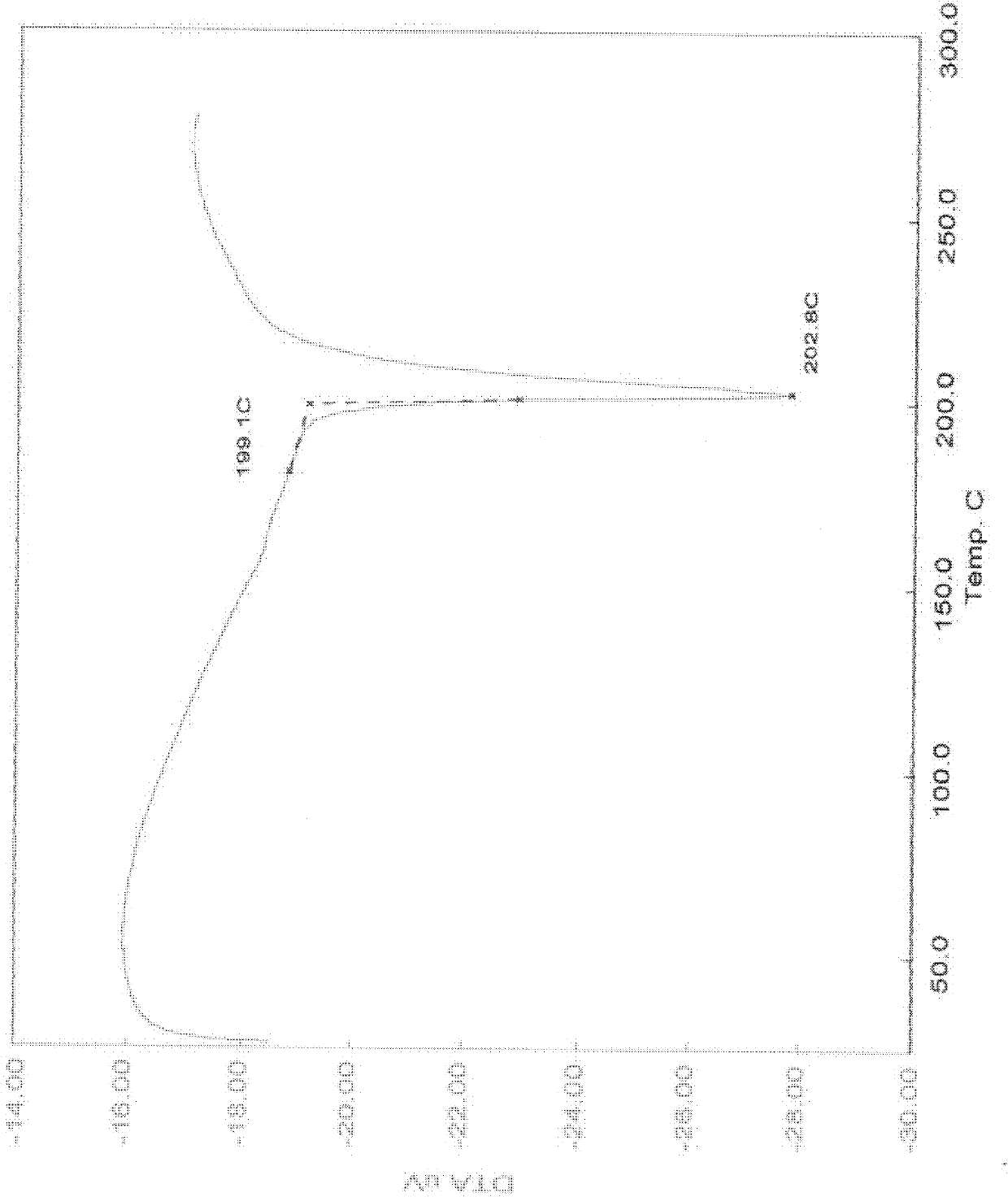
[0059] Melting point: 200.0-203.0°C; product purity measured by HPLC: 99.4765%; crystal drying weight loss...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com