Application of compound 6-O-angeloylplenolin to pharmacy
A technology of natural compounds and compositions, applied in the direction of active ingredients of boron compounds, active ingredients of heavy metal compounds, drug combinations, etc., can solve problems such as unclear synergistic effects, achieve good killing effect, high recurrence rate, and large toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
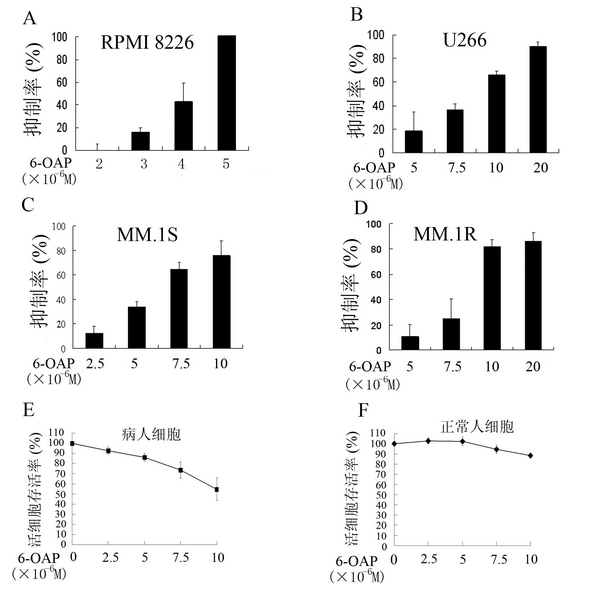
[0041] With different concentrations of natural compound 6-OAP (2 × 10 -6 -5×10 -6 M) Treat RPMI8226 cells, add 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfonic acid benzene after 44 hours )-2H-tetrazolium monosodium salt (WST-8) CCK-8 detection reagent, incubated for another 4 hours, and then measured its absorbance at 450 nm (OD450) with a microplate reader. It was found that 6-OAP treatment can significantly reduce the OD450 of RPMI8226 cells, and 6-OAP can significantly inhibit the proliferation of RPMI8226 cells, and the inhibition rate is positively correlated with the drug concentration (see figure 1 A). According to the growth inhibitory effect of 6-OAP on RPMI8226 cells, the half inhibitory concentration (IC) of 6-OAP on RPMI8226 cells was calculated 50 ) for 3.5 x 10 -6 M.
Embodiment 2
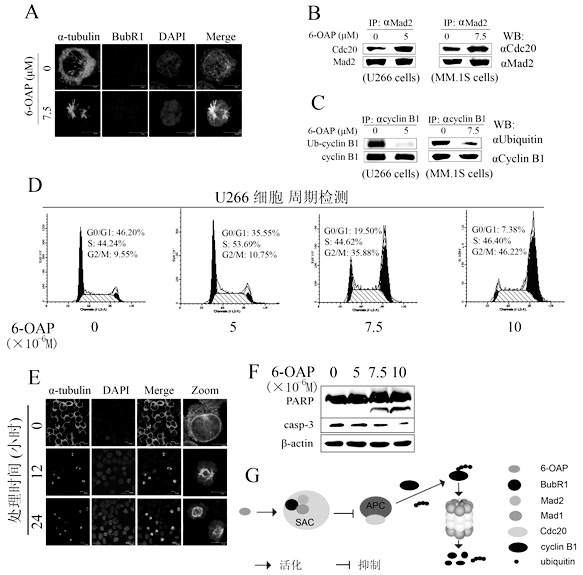
[0043] Use 7.5×10 -6 6-OAP of M treated U266 cells, detected the spindle checkpoint protein BubR1 by immunofluorescence after 24 hours, and found that 6-OAP promoted the aggregation of BubR1, indicating that the spindle checkpoint was activated ( figure 2 A). Furthermore, the Cdc20 bound to the spindle checkpoint protein Mad2 was detected by co-immunoprecipitation method, and it was found that the activation of the spindle checkpoint by 6-OAP can promote the binding of Cdc20 ( figure 2 B), Cdc20 is the binding activator of APC ubiquitin ligase, which will make APC lose its ubiquitination activity after being bound by spindle checkpoint protein. Therefore, with 7.5×10 -6 U266 and MM1S cells were treated with 6-OAP of M, and the ubiquitination activity of cyclin B1 was detected by immunoprecipitation after 24 hours, and it was found that the ubiquitination of cyclin B1 in 6-OAP-treated cells was reduced ( figure 2 C), showing that cyclin B1 degradation is inhibited. Wit...
Embodiment 3
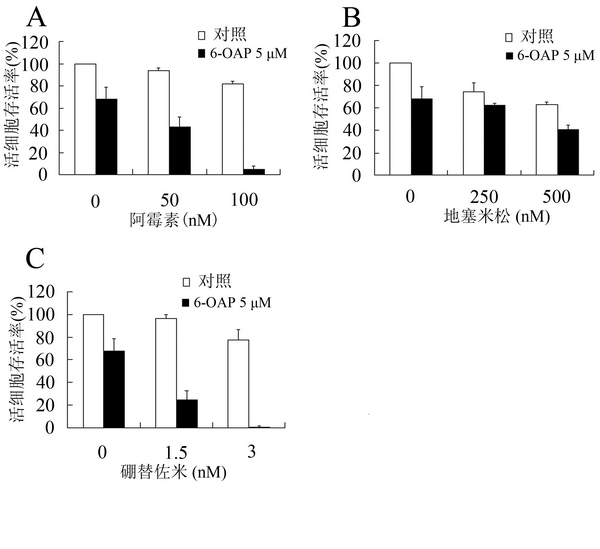
[0045] After MM.1S cells were pre-attached for 24 hours, different concentrations (5×10 -6 -7.5×10 -6 M) MM.1S cells were treated with 6-OAP combined with doxorubicin, dexamethasone, and bortezomib. After 44 hours, MTT detection reagent was added to incubate for another 4 hours, and then the absorbance value at 570 nm was measured with a microplate reader (OD570). It was found that 6-OAP combined with doxorubicin ( image 3 A), dexamethasone ( image 3 B) and bortezomib ( image 3 C) Treatment can significantly reduce the OD570 of MM.1S cells, and 6-OAP combined with conventional drugs has a better effect on the treatment of MM.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Half inhibitory concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com