Method for preparing lithium ion battery cathode material coated iron sesquioxide
A technology of ferric oxide and lithium-ion batteries, which can be used in battery electrodes, circuits, electrical components, etc., and can solve problems that have not been reported in the literature.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
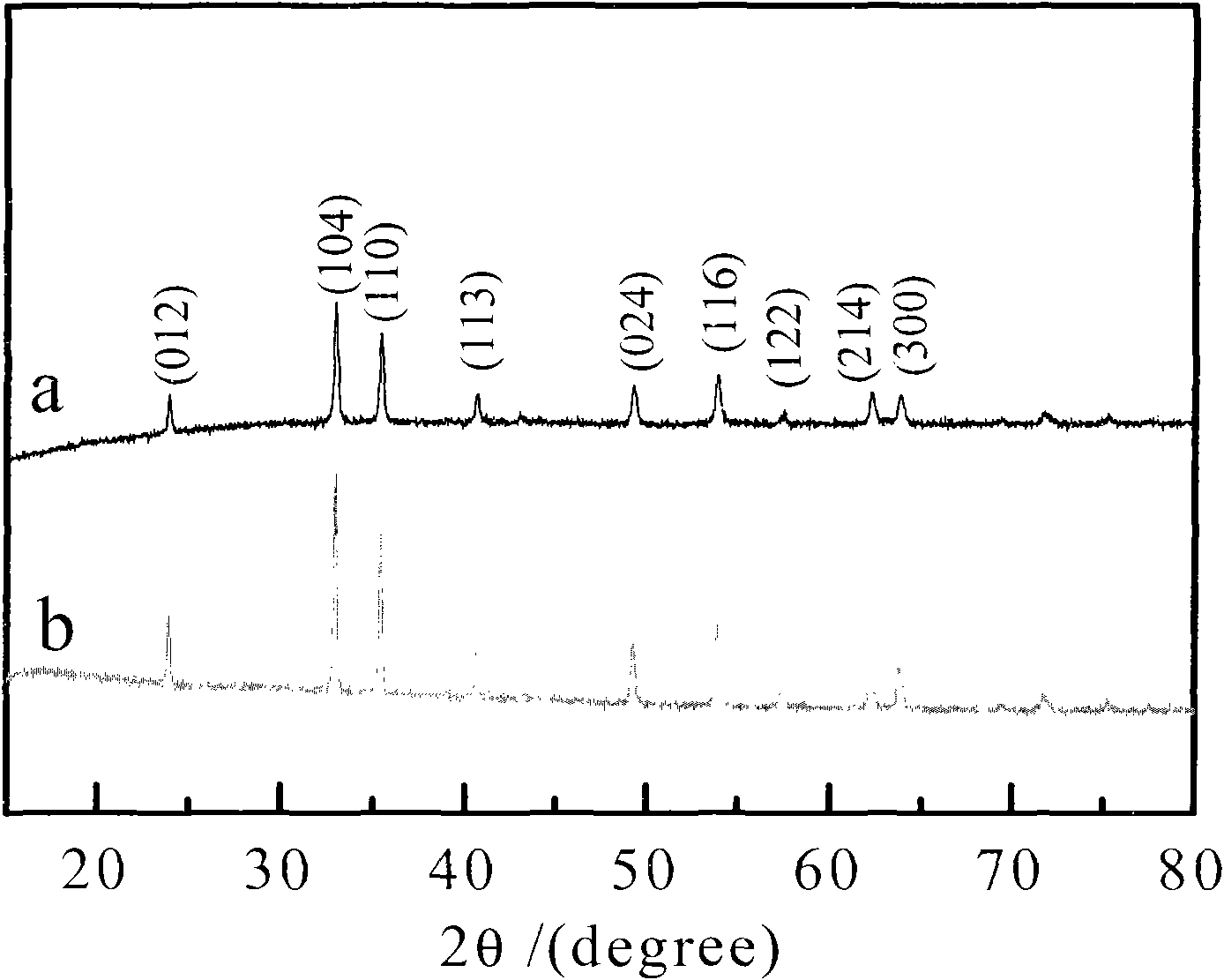
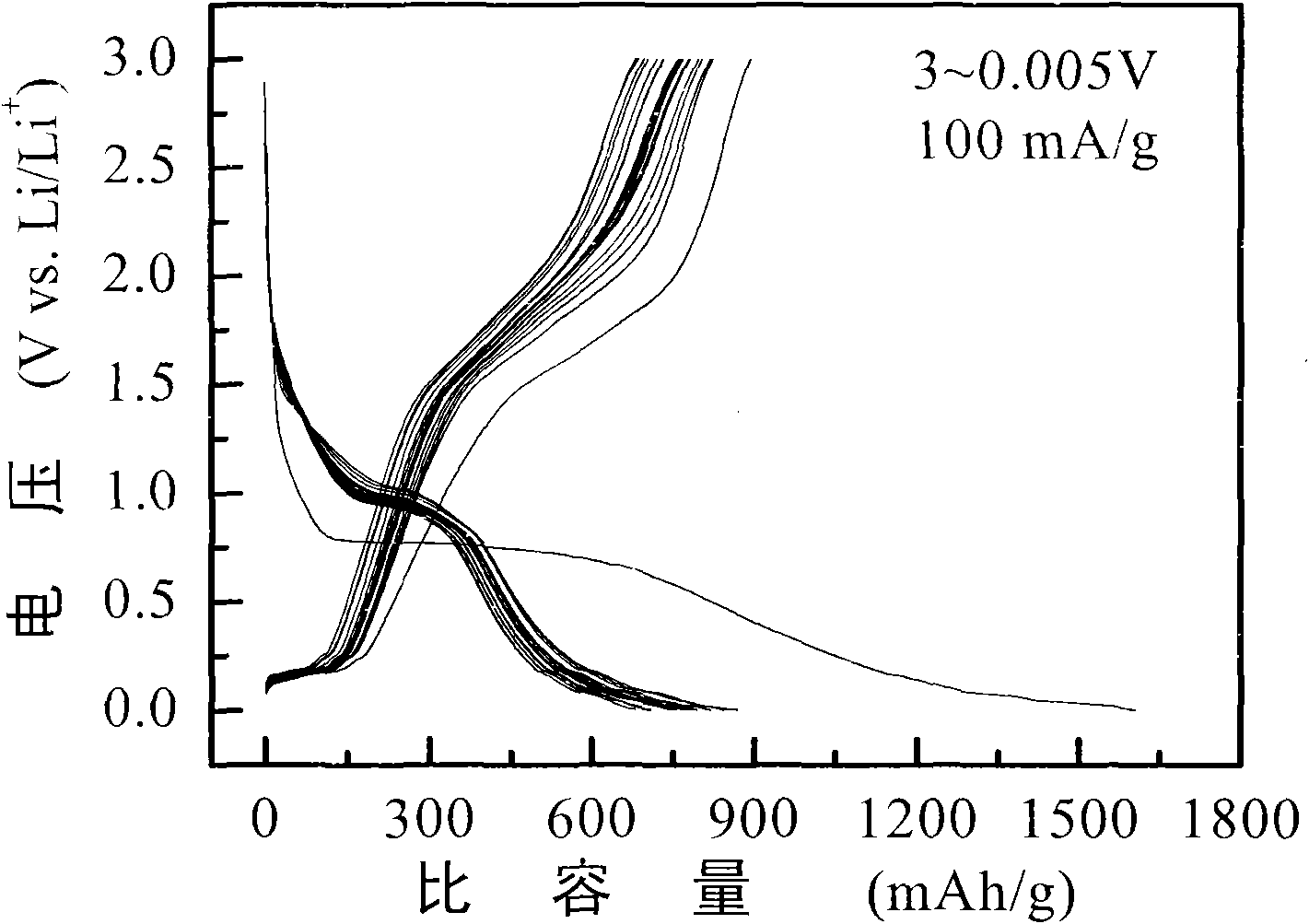
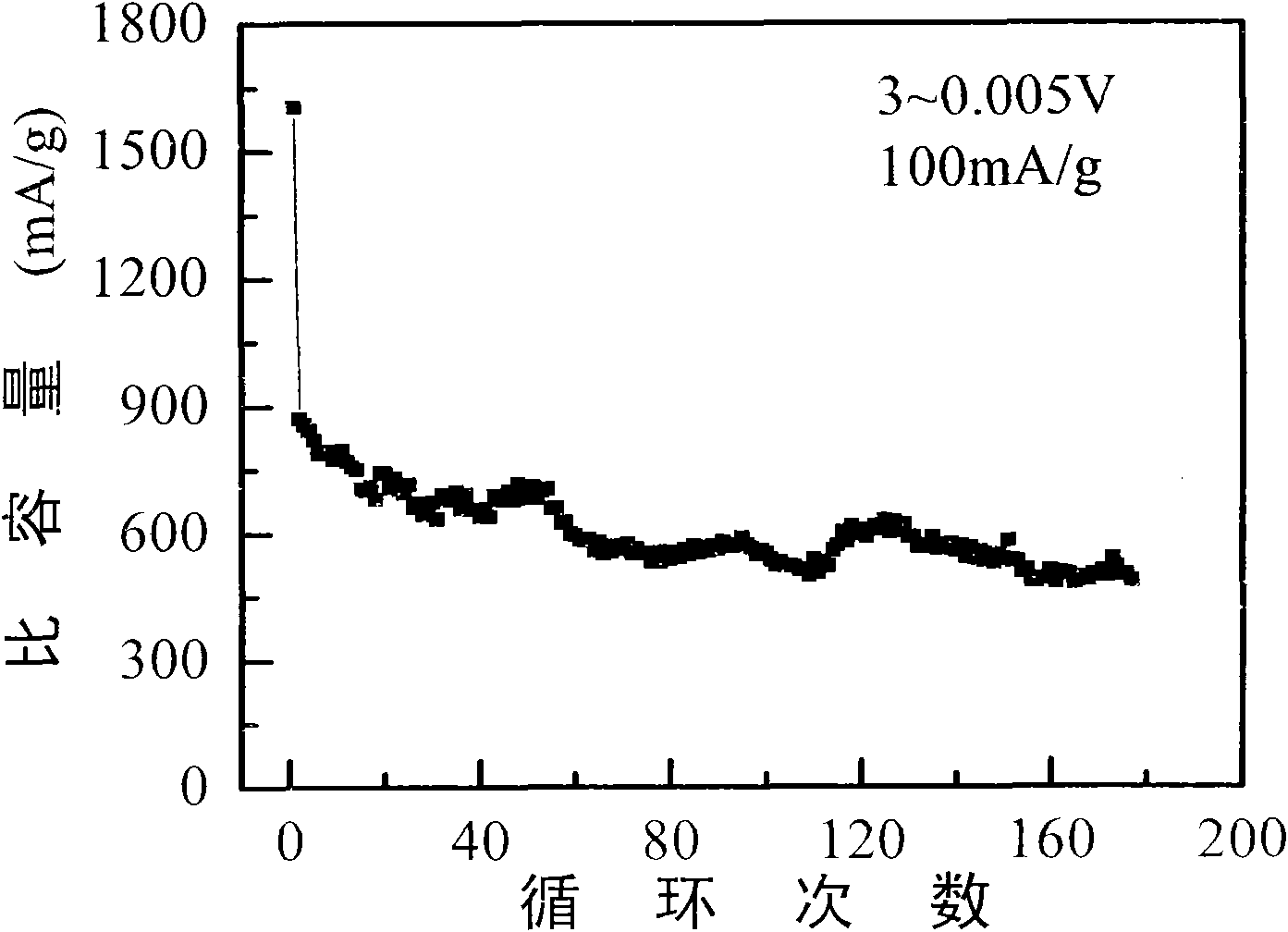
[0020] 5 g of ferric chloride hexahydrate and 0.5 g of sucrose were added to 400 ml of deionized water, and ultrasonically oscillated for 0.5 hour to obtain a uniform mixed solution. Put the above mixed solution into a round bottom flask, stir for 30 minutes, and gradually add sodium hydroxide to adjust the pH of the solution to 6. The mixed solution was transferred to the bottom of the hydrothermal reaction tank and reacted at 160° C. for 12 hours. The hydrothermal product is centrifugally cleaned several times with deionized water and absolute ethanol respectively, and after being vacuum freeze-dried, a carbon precursor-coated iron sesquioxide composite material is obtained. The composite was calcined at 600°C under an argon atmosphere for 2 hours and cooled to room temperature to obtain Fe 2 o 3 @C Composite.
[0021] figure 1 a is the Fe obtained in Example 1 2 o 3 XRD patterns of @C composites. From the diffraction peaks in the figure, it can be seen that the compo...
Embodiment 2
[0025] Add 5 g of ferric chloride hexahydrate and 1.0 g of glucose into 500 ml of deionized water, and ultrasonically oscillate for 0.5 hour to obtain a uniform mixed solution. Put the above mixed solution into a round bottom flask, stir for 30 minutes, gradually add ammonia water, and adjust the pH of the solution to 9. The mixed solution was transferred to the bottom of the hydrothermal reaction tank, and reacted at 180° C. for 24 hours. The hydrothermal product is centrifugally cleaned several times with deionized water and absolute ethanol respectively, and after being vacuum freeze-dried, a carbon precursor-coated iron sesquioxide composite material is obtained. The composite was calcined at 800°C under an argon atmosphere for 4 hours and cooled to room temperature to obtain Fe 2 o 3 @C Composite.
[0026] figure 1 b is the Fe obtained in Example 2 2 o 3 XRD patterns of @C composites. From the diffraction peaks in the figure, it can be seen that the composite mainl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com