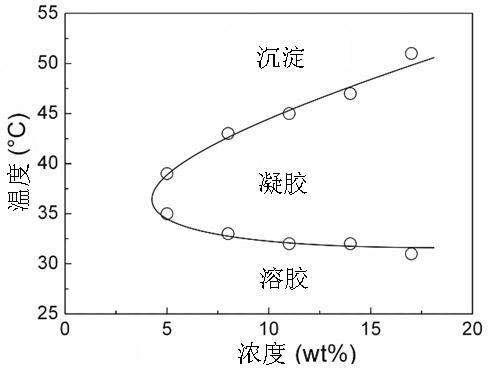
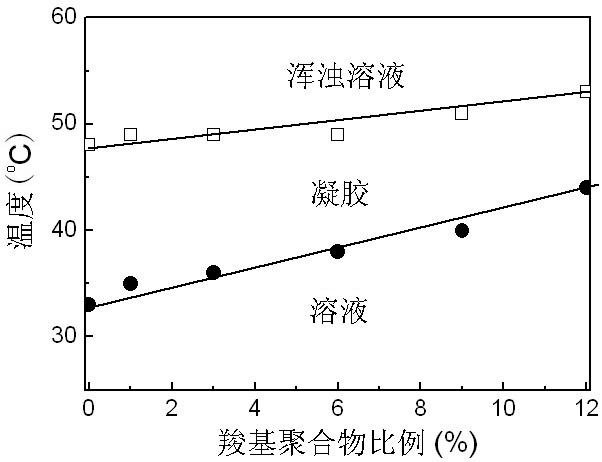
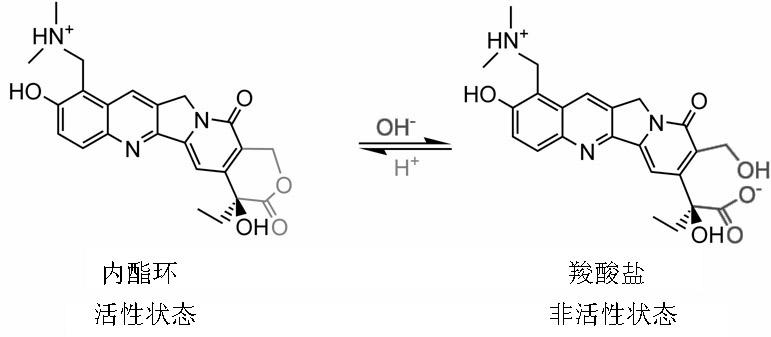
Gel sustained-release preparation capable of improving ring-close rates of camptothecin and derivative lactone ring thereof
A slow-release preparation, camptothecin technology, applied in the field of medicine, can solve the problems of loss of anti-cancer activity, insufficient closed-loop rate, and it is difficult to load hydrophobic drugs into it, so as to enhance the activity, reduce the drug dosage, and improve the closed-loop rate. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1 In a 500 ml three-necked flask, add 30 g of dihydroxy polyethylene glycol (1500), remove water under vacuum at 130 °C for 4 hours, cool to 70 °C with argon, add 60 g of lactide, 15 g of glycolide, and 26 mg of octanoic acid Stannous (containing a small amount of toluene), evacuated at 100 °C for 30 minutes, passed argon, heated to 130 °C and reacted for 12 hours. After the reaction was completed, the product was poured out while hot, and after cooling, the product was dissolved in dichloromethane, and ether was precipitated to obtain the desired gel material.
Embodiment 2
[0061] Example 2 In a 500 ml three-necked flask, add 30 g of dihydroxy polyethylene glycol (1500), remove water under vacuum at 130 °C for 4 hours, cool to 70 °C with argon, add 48 g of lactide, 26 mg of stannous octoate (containing a small amount of water) Toluene), evacuated at 100 °C for 30 minutes, passed argon, heated to 130 °C and reacted for 12 hours. After the reaction was completed, the product was poured out while hot, and after cooling, the product was dissolved in dichloromethane, and ether was precipitated.
[0062] Take 15 g of the above materials, add 4 g of succinic anhydride, and 2 mL of pyridine, dissolve them in 100 mL of dichloromethane, and reflux for 48 hours. Most of the dichloromethane and pyridine were removed by suspension, washed with 80°C hot water (add a few drops of hydrochloric acid), pyridine and dichloromethane were removed, and freeze-dried to obtain a gel material with a carboxyl end group.
Embodiment 3
[0063] Example 3 In a 500 ml three-necked flask, add 30 g of dihydroxy polyethylene glycol (1500), dewater at 130 °C for 4 hours, cool to 70 °C with argon, add 34 g of lactide, 34 g of caprolactone, and 26 mg of octanoic acid Stannous (containing a small amount of toluene), evacuated at 100 °C for 30 minutes, passed argon, heated to 130 °C and reacted for 12 hours. After the reaction was completed, the product was poured out while hot, and after cooling, the product was dissolved in dichloromethane, and ether was precipitated to obtain the desired gel material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com