Synthesizing method of polyimide
A polyimide and synthesis method technology, applied in the field of polymer material synthesis, can solve the problems of high recycling cost, organic solvent volatility and harm to the environment and operators, and achieve easy recycling, easy recycling, and recycling high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1, the preparation of polyimide
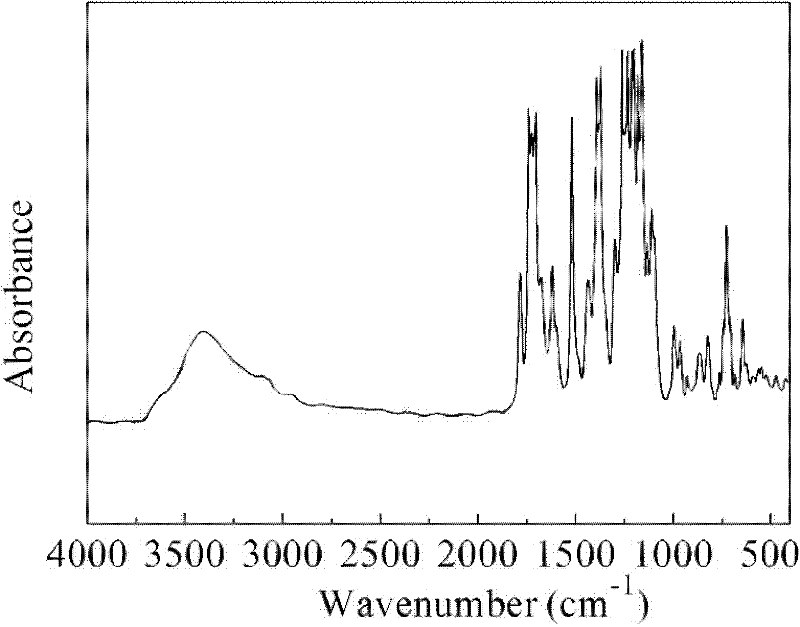
[0020] Add 52.002 g of 1-butyl-3-methylimidazolium tetrafluoroborate ionic liquid into a dry four-necked flask equipped with a mechanical stirrer, a thermometer, and a nitrogen gas inlet and outlet tube, raise the temperature to 60 ° C, and protect it with nitrogen gas. Under mechanical stirring, add 9.157g (25mmol) of 2,2-bis(3-amino-4-hydroxyphenyl)hexafluoropropane (Bis-AP-AF), stir until completely dissolved and then add 8.177g (25.375mmol ) of 3,3',4,4'-benzophenonetetraacid dianhydride (BTDA), wherein, 2,2-bis(3-amino-4-hydroxyphenyl)hexafluoropropane and 3,3', The molar ratio of 4,4'-benzophenone tetraacid dianhydride is 1:1.015; 2,2-bis(3-amino-4-hydroxyphenyl)hexafluoropropane and 3,3',4,4'- The total mass of benzophenone tetraacid dianhydride accounts for 25% of the mass percentage of 1-butyl-3-methylimidazolium tetrafluoroborate ionic liquid. After the dissolution is complete, the temperature is raised to 200 ° C ...
Embodiment 2
[0021] Embodiment 2, the preparation of polyimide
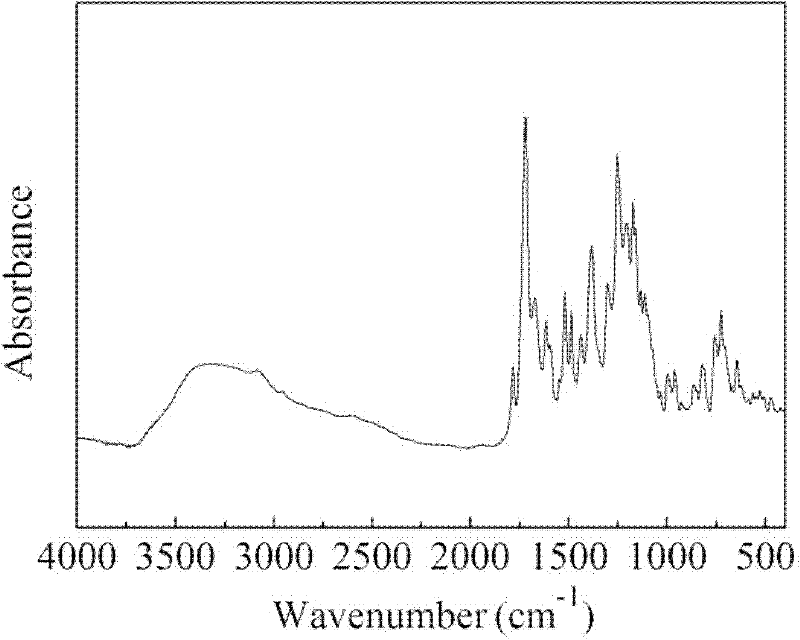
[0022] The method of this embodiment is the same as that of Example 1, except that the ionic liquid is changed to 1-butyl-3-methylimidazolium hexafluorophosphate; a yellow solid powder is finally obtained, and its infrared absorption spectrum is as follows: figure 2 shown by figure 2 (imino and carbonyl characteristic absorption peaks) it can be seen that the corresponding polyimide target product is obtained by polymerization, and Mn is 0.817 × 10 4 , The yield of this product is 94%.
Embodiment 3
[0023] Embodiment 3, the preparation of polyimide
[0024] The method of this embodiment is the same as that of Example 1, except that the temperature of the polymerization reaction is changed to 180° C., the time of the polymerization reaction is changed to 6 hours, and finally a yellow solid powder is obtained, and its infrared absorption spectrum is the same as that of figure 1 Similar; 95% yield of this product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com