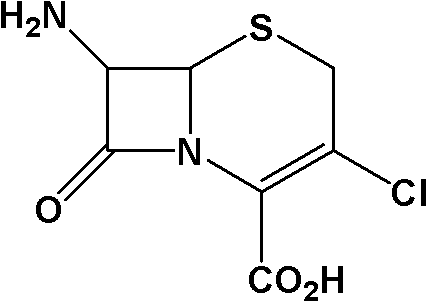
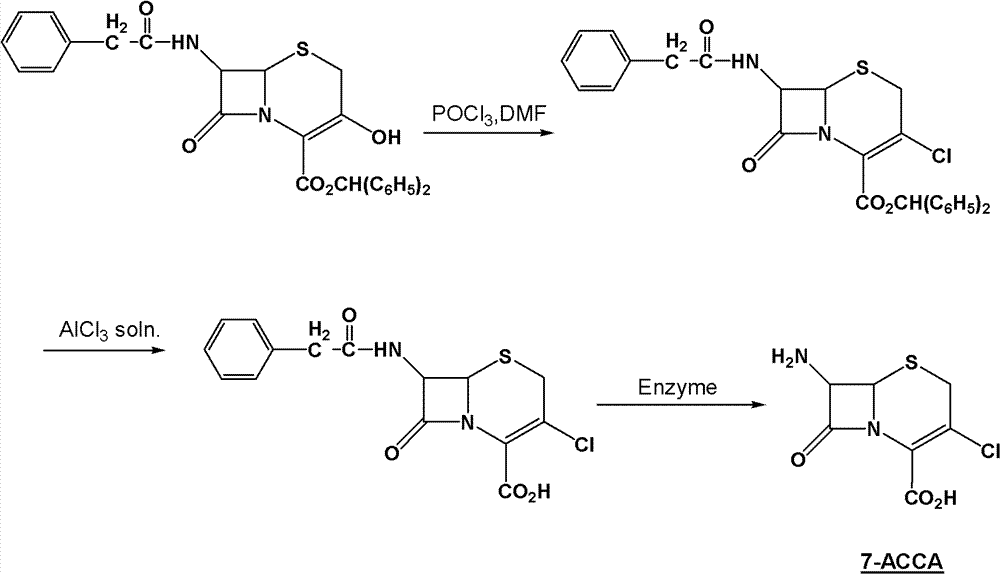
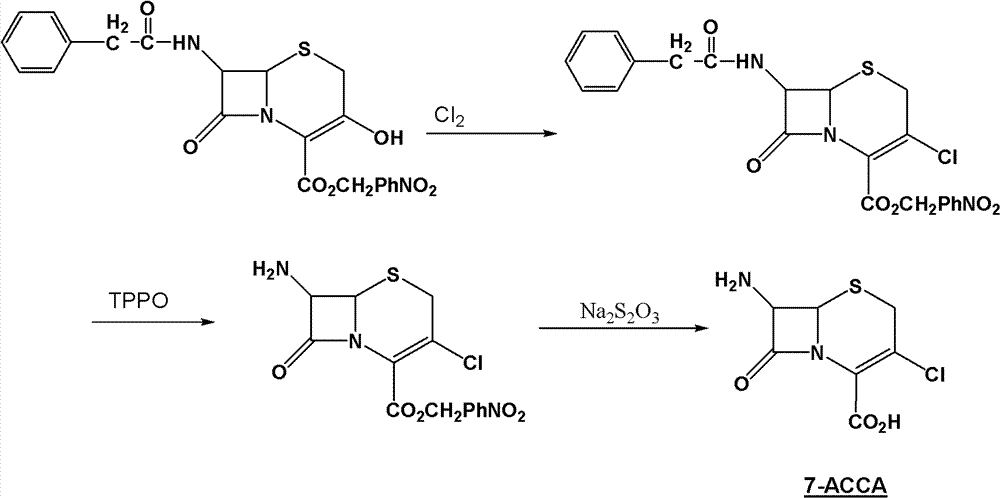
Method for preparing 7-ACCA
A technology for controlling temperature and phenylacetamide, applied in the field of preparation of 7-ACCA
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1) Preparation of 7-phenylacetamido-3-chloro-3-cephalosporin-4-carboxylic acid p-nitrobenzyl ester:
[0032] Take 400g of chloroform, 68g of triphosgene (triphosgene, chemical name, bis(trichloromethyl)carbonate, referred to as BTC), 72g of DMF, control the temperature at 0±1°C, stir for 2 hours, add 60g 7-phenylacetamido-3-hydroxyl-3-cephalosporin-4-carboxylic acid p-nitrobenzyl ester, controlled temperature 55±2°C, stirred and reacted for 10 hours, and slowly poured into 150g (10 %, mass percentage concentration) in NaOH solution, control pH 12~13, control temperature 25 ± 2 ℃, stir reaction for 1 hour, stand still, separate layers, distill off chloroform under reduced pressure, add methanol 150g, cool and crystallize, filter, After washing and drying, 54.7 g of p-nitrobenzyl 7-phenylacetamido-3-chloro-3-cephalosporin-4-carboxylate was obtained, with a purity of more than 98%;
[0033] 2) Preparation of 7-phenylacetamido-3-chloro-3-cephalosporin-4-carboxylic acid:
...
Embodiment 2
[0038] 1) Preparation of 7-phenylacetamido-3-chloro-3-cephalosporin-4-carboxylic acid p-nitrobenzyl ester:
[0039] Take 5000g of chloroform, 800g of triphosgene (triphosgene, chemical name, bis(trichloromethyl)carbonate, referred to as BTC), 1000g of DMF, control the temperature at 0±1°C, stir for 3 hours, add 600g 7-phenylacetamido-3-hydroxyl-3-cephalosporin-4-carboxylic acid p-nitrobenzyl ester, controlled temperature 55±2°C, stirred and reacted for 12 hours, and slowly poured into 2000g (10 %, mass percentage concentration) in NaOH solution, control pH 12~13, control temperature 25 ± 2 ℃, stir reaction for 1 hour, stand still, separate layers, distill off chloroform under reduced pressure, add methanol 2000g, cool and crystallize, filter, Washing and drying to obtain 550 g of p-nitrobenzyl 7-phenylacetamido-3-chloro-3-cephalosporin-4-carboxylate product with a purity of more than 98%;
[0040] 2) Preparation of 7-phenylacetamido-3-chloro-3-cephalosporin-4-carboxylic acid:...
Embodiment 3
[0045] 1) Preparation of 7-phenylacetamido-3-chloro-3-cephalosporin-4-carboxylic acid p-nitrobenzyl ester:
[0046] Take 2000g of chloroform, 430g of triphosgene (triphosgene, chemical name, bis(trichloromethyl)carbonate, referred to as BTC), 450g of DMF, control the temperature at 0±1°C, stir for 3 hours, add 300g 7-phenylacetamido-3-hydroxyl-3-cephalosporin-4-carboxylic acid p-nitrobenzyl ester, controlled temperature 55±2°C, stirred and reacted for 12 hours, and slowly poured into 1000g (10 %, mass percent concentration) in NaOH solution, control pH 12~13, control temperature 25 ± 2 ℃, stir reaction for 1 hour, stand still, separate layers, distill off chloroform under reduced pressure, add methanol 700g, cool and crystallize, filter, After washing and drying, 270 g of p-nitrobenzyl 7-phenylacetamido-3-chloro-3-cephalosporin-4-carboxylate was obtained, with a purity of more than 98%;
[0047] 2) Preparation of 7-phenylacetamido-3-chloro-3-cephalosporin-4-carboxylic acid: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com