Vitexin glucoside injection and preparation method thereof
A technology of vitexin glucoside and glucoside injection, which is applied in the fields of medical formula, medical preparations containing active ingredients, drug delivery, etc., and can solve the problems of low bioavailability, difficult control of product quality, and slow onset of hawthorn leaf preparations and other issues, to achieve the effect of high bioavailability, easy industrial production and application, and fast onset of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
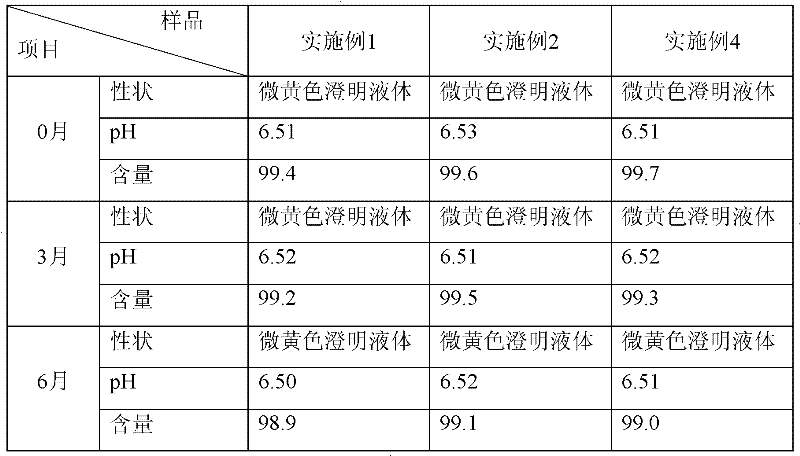
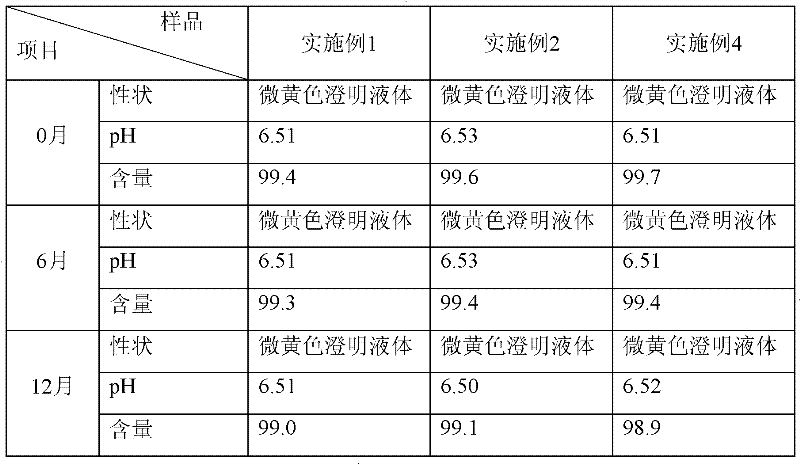
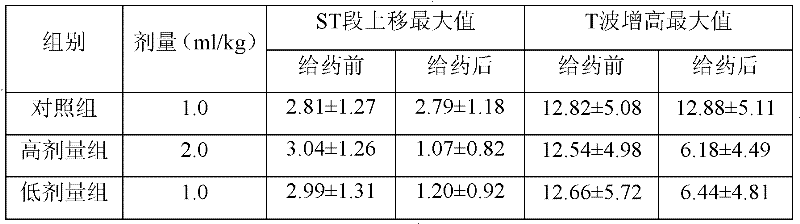
[0014] Take 10 grams of vitexin glucoside, 0.5 grams of sodium bisulfite, add 8 liters of water for injection to dissolve, adjust the pH to 6.5 with 10% sodium hydroxide solution, add 8 grams of activated carbon, stir well, decarbonize and filter, and filter the filtrate. 0.22 micron filter membrane, add water for injection to 10 liters, potting, and sterilize in a water bath at 100°C for 30 minutes to obtain 1000 vials of vitexin glucoside injection with a specification of 10ml: 10mg.
Embodiment 2
[0016] Take 50 grams of vitexin glucoside, 2.5 grams of sodium bisulfite, add 8 liters of water for injection to dissolve, adjust the pH to 6.5 with 10% sodium hydroxide solution, add 8 grams of activated carbon, stir well, decarbonize and filter, and filter the filtrate. 0.22 micron filter membrane, add water for injection to 10 liters, potting, and sterilize in a water bath at 100°C for 30 minutes to obtain 1000 vials of vitexin glucoside injection with a specification of 10ml: 50mg.
Embodiment 3
[0018] Take 50 grams of vitexin glucoside, 2.5 grams of sodium pyrosulfite, add 8 liters of water for injection to dissolve, adjust the pH to 6.5 with 10% sodium hydroxide solution, add 8 grams of activated carbon, stir well, decarbonize and filter, and the filtrate passes through 0.22 microns add water for injection to 10 liters, potting, and sterilize in a water bath at 100°C for 30 minutes to obtain 1000 vials of vitexin glucoside injection with a specification of 10ml: 50mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com