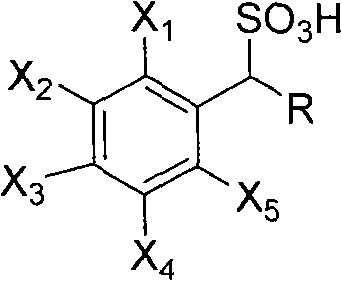
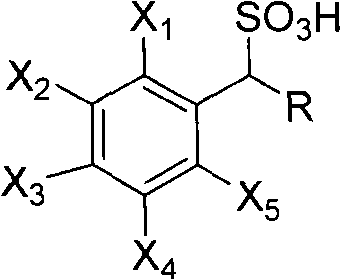
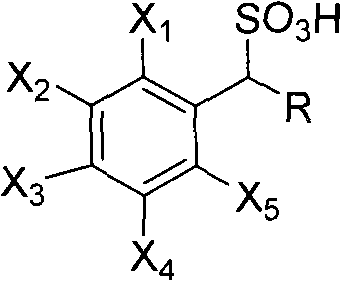
Method for preparing photoactived amino acid through resolution
An amino acid and photoactivation technology, applied in organic chemistry methods, cyanide reaction preparation, chemical instruments and methods, etc., can solve the undiscovered AES combined chiral reagent resolution, the first separation of diastereomer product yield And problems such as low optical purity, resolution product yield and optical purity, etc., to achieve the effect of high resolution efficiency, high purity and high recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] (1) In 3500mL aqueous solution, add 167.2g of racemic p-hydroxyphenylglycine, 186.8g of combined AES resolving agent is heated to reflux until the solution is clear, after stirring for 5 hours, it is naturally cooled to 68°C, crystals are precipitated, filtered, and 6mL The filter cake was washed with ice water, rinsed with 2 mL of methanol, and dried to obtain 174.5 g of the filter cake.
[0023] The main resolving agent in the combined AES resolving agent is (+)-1-phenylethanesulfonic acid, and the auxiliary resolving agent is 1-(4'-bromophenyl)-ethanesulfonic acid, the molar ratio of the two is It is 99:1.
[0024] (2) Dissolve the above filter cake into 500mL methanol / water solution (volume ratio is 1:1), stir, add 10% NaOH dropwise to the pH of the solution = 5.4, stir for 1h, let stand for 1h, filter, and use for filter cake Rinse with a small amount of ice water and dry to obtain 81.4 g of D-(-)-p-hydroxyphenylglycine with a yield of 97.4%. [α] 20 D -158.0° (...
Embodiment 2
[0031] (1) In 3000mL aqueous solution, add 167.2g of racemic p-hydroxyphenylglycine, 195g of combined AES resolving agent is heated to reflux until the solution is clear, after stirring for 5h, it is naturally cooled to 65°C, crystals are precipitated, filtered, and washed with 6mL of ice The filter cake was washed with water, rinsed with 2 mL of methanol, and dried to obtain 173.7 g of the filter cake.
[0032] The main resolving agent in the described combined AES resolving agent is (-)-1-phenylethanesulfonic acid, and the auxiliary resolving agent is 1-(4'-nitrophenyl)-ethanesulfonic acid, both moles The ratio is 80:20.
[0033](2) Dissolve the above filter cake into 510mL methanol / water solution (volume ratio is 1:1), stir, add 10% KOH dropwise to the pH of the solution=5.4, stir for 1h, let stand for 1h, filter, and use for filter cake Rinse with a small amount of ice water and dry to obtain 81.7 g of L-(+)-p-hydroxyphenylglycine with a yield of 97.8%. [α] 20 D +157.7...
Embodiment 3
[0040] (1) In 3500mL aqueous solution, add 167.2g of racemic p-hydroxyphenylglycine, 189.1g of combined AES resolving agent is heated to reflux until the solution is clear, after stirring for 5h, it is naturally cooled to 70°C, crystals are precipitated, filtered, and 5mL The filter cake was washed with ice water, rinsed with 1 mL of methanol, and dried to obtain 174.2 g of the filter cake.
[0041] The main resolving agent in the combined AES resolving agent is (-)-1-phenylethanesulfonic acid, and the auxiliary resolving agent is 1-(4'-chlorophenyl)-ethanesulfonic acid, the molar ratio of the two is It is 91:9.
[0042] (2) Dissolve the above filter cake into 480mL methanol / water solution (1:9 by volume), stir, and add Na 2 CO 3 Solution to pH=5.4, stirred for 1 h, allowed to stand for 1 h, filtered, rinsed the filter cake with a small amount of ice water, and dried to obtain 80.5 g of L-(+)-p-hydroxyphenylglycine with a yield of 96.3%. [α] 20 D +157.3° (c=1, 1N HCl), op...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com