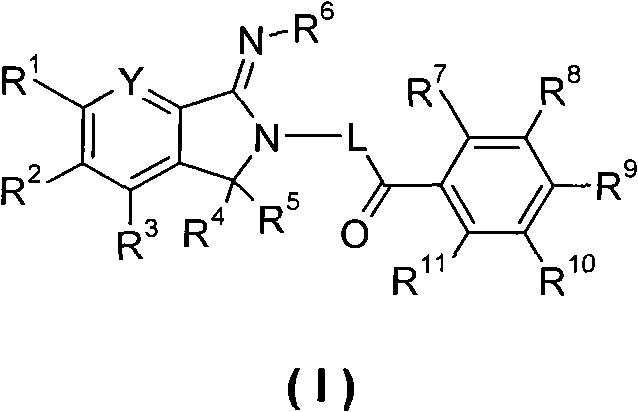
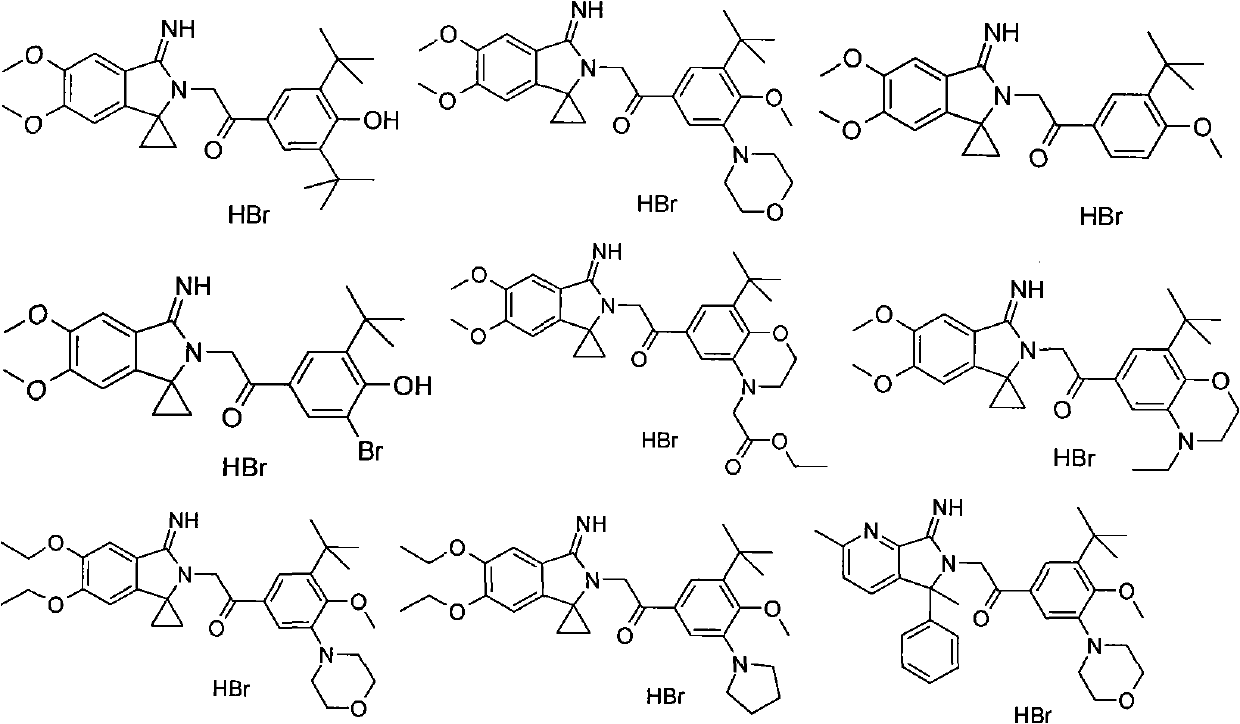
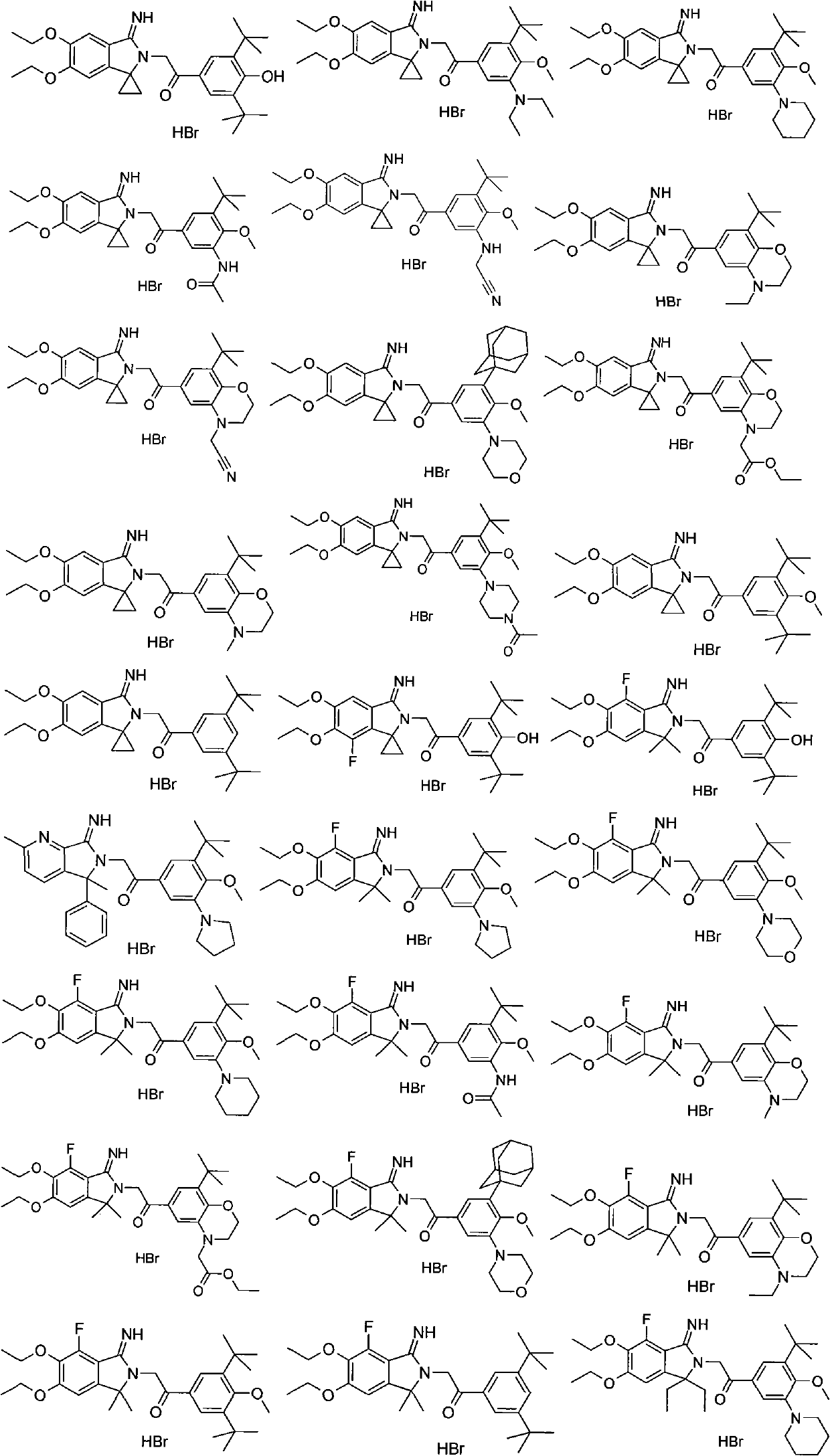
5,5-disubstituted-2-iminopyrrolidine derivatives, preparation method thereof, and medical applications thereof
An alkyl and aryl technology, applied in the field of 5,5-disubstituted-2-iminopyrrolidine derivatives, its preparation and its application in medicine, can solve the problem of increased bleeding, weakened and disturbed platelets Aggregation process etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0184] 1-(3,5-di-tert-butyl-4-hydroxyphenyl)-2-(3′-imino-5′,6′-dimethoxy-spiro[cyclopropane-1,1′-iso indole
[0185] Phenyl]-2'-yl)ethanone hydrobromide
[0186]
[0187] first step
[0188] 4,5-Dimethoxyphthalonitrile
[0189] Dissolve 1,2-dibromo-4,5-dimethoxybenzene 1a (20.01g, 68mmol) in 100mL N,N-dimethylformamide, add cuprous cyanide (24.00g, 272mmol), The reaction was stirred at 150°C for 1 hour, and continued at 170°C for 5 hours. The reaction solution was poured into 100 mL of ammonia water, 400 mL of ethyl acetate was added, filtered, and the filter cake was washed with ethyl acetate (100 mL×6). The filtrate was extracted with ethyl acetate (200mL×2), the organic phases were combined, dried over anhydrous sodium sulfate, filtered, the filtrate was concentrated under reduced pressure, and the crude product was purified by recrystallization (methanol:ethyl acetate=10mL:20mL) to obtain the t...
Embodiment 2
[0208] 1-(3-tert-butyl-4-methoxy-5-morpholine phenyl)-2-(3'-imino-5',6'-dimethoxy-spiro[cyclopropane-1, 1′-isoindole
[0209] Indoline]-2'-yl)ethanone hydrobromide
[0210]
[0211]
[0212] first step
[0213] 1-(3-tert-butyl-4-hydroxyphenyl)ethanone
[0214] Under a dry ice-acetone bath, dissolve aluminum chloride (71.0 g, 0.53 mol) in 250 mL of dichloromethane, add 2-tert-butyl-phenol 2a (81.6 mL, 0.53 mol), stir the reaction for 2 hours, add ethyl alcohol dropwise Acid chloride 2b (37.9 mL, 0.53 mol), continued stirring for 1 hour. 300 mL of ice water was added to the reaction liquid, filtered, and the solid was dried in vacuo to obtain the title product 1-(3-tert-butyl-4-hydroxyphenyl)ethanone 2c (32 g, white solid), yield: 31.3%.
[0215] MS m / z(ESI): 191[M-1]
[0216] second step
[0217] 1-(3-tert-butyl-4-hydroxy-5-iodo-phenyl)ethanone
[021...
Embodiment 3
[0244] 1-(3-tert-butyl-4-methoxyphenyl)-2-(3'-imino-5',6'-dimethoxy-spiro[cyclopropane-1,1'-isoind indole
[0245] Phenyl]-2'-yl)ethanone hydrobromide
[0246]
[0247] first step
[0248] 1-(3-tert-butyl-4-methoxyphenyl)ethanone
[0249] Dissolve 1-(3-tert-butyl-4-hydroxyphenyl)ethanone 2c (6.3 g, 32.8 mmol) in 50 mL of acetone, add potassium carbonate (13.6 g, 98.4 mmol) and iodomethane (11.65 g, 82 mmol ), stirred and reacted at 50°C for 2 hours. Filtrate, concentrate the filtrate under reduced pressure, add 50mL water and 50mL dichloromethane, separate the layers, wash the organic phase with saturated sodium chloride solution (20mL×3), dry over anhydrous sodium sulfate, filter, and concentrate the filtrate under reduced pressure to obtain the title product 1-(3-tert-butyl-4-methoxyphenyl)ethanone 3a (6.0 g, white solid), yield: 88.6%.
[0250] 1 H NMR (400MHz, CDCl 3 , ppm): δ7.99 (d, J=2....
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com