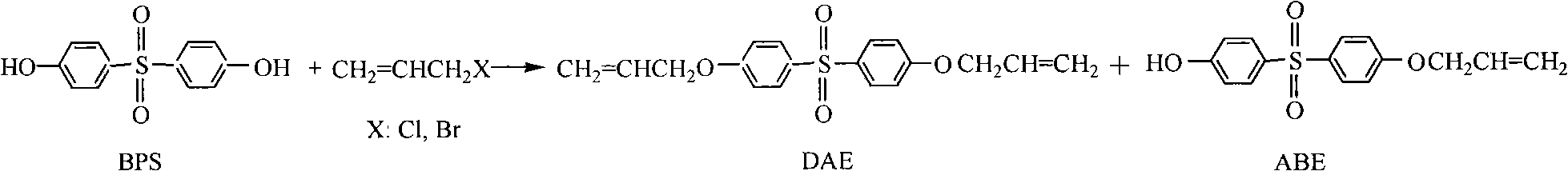
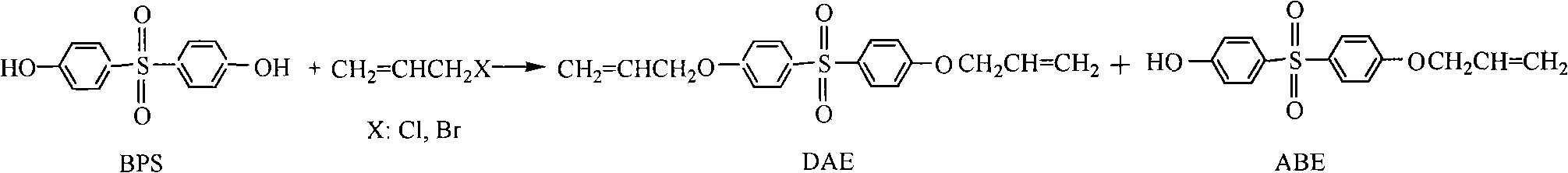
Method for preparing bisphenol S allyl ether
A technology of bisallyl ether and allyl ether, which is applied in the field of preparation of aromatic sulfone organic compounds, can solve the problems that are not conducive to the improvement of single-line production capacity and economic benefits, slow reaction speed, waste alkali pollution, etc., and achieve Easy to control, high product yield, environment-friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] In a 50L reactor, add 20kg of bisphenol S, add 20% sodium hydroxide solution until the pH value is 10.7, stir, heat to dissolve, add 0.2kg of tetrabutylammonium bromide, slowly drop 2.5kg of allyl chloride, Heat to reflux (60°C) for 5 hours. Filter after cooling to recover tetrabutylammonium bromide. After the filtrate is statically stratified, liquid separation is carried out, the upper layer is the organic phase, and the lower layer is the aqueous phase. The pH of the aqueous phase was adjusted to 9.1 with 60% sulfuric acid solution, and solids were precipitated. The crude product of ABE was obtained by filtration, and purified by recrystallization with 5 kg of 25% ethanol. The solid product obtained after drying was ABE (4.8 kg). The organic phase was washed with water until neutral, heated to reflux, filtered after cooling to obtain a solid, and DAE (15.7kg) was obtained after drying. Filtrate recovery.
Embodiment 2
[0035] The tetrabutylammonium bromide reclaimed in embodiment 1 is tested again by the scheme of embodiment 1, obtains 15.2kg DAE and 5.1kg ABE.
Embodiment 3
[0037] Cetyltrimethylammonium chloride was used to replace tetrabutylammonium bromide in Example 1, and the test was carried out according to the scheme of Example 1 to obtain 15.7kg DAE and 4.9kg ABE.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com