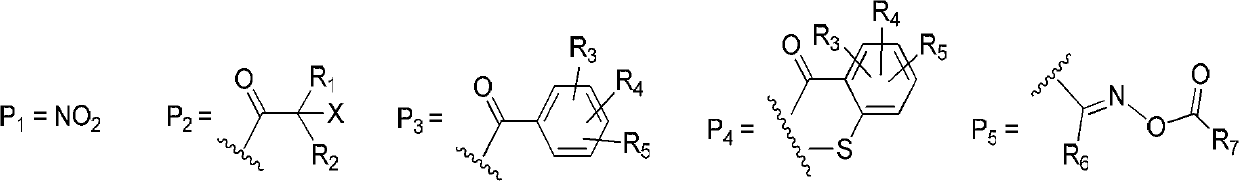
Carbazole cyclic derived type aromatic ketone compound as well as preparation method and photo initiator thereof
A carbazole ring and compound technology, applied in the field of photoinitiators, can solve the problems of low residual odor and need to be improved, and achieve the effects of reducing residual odor, enhancing photoinitiating activity and photocrosslinking degree, and improving solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
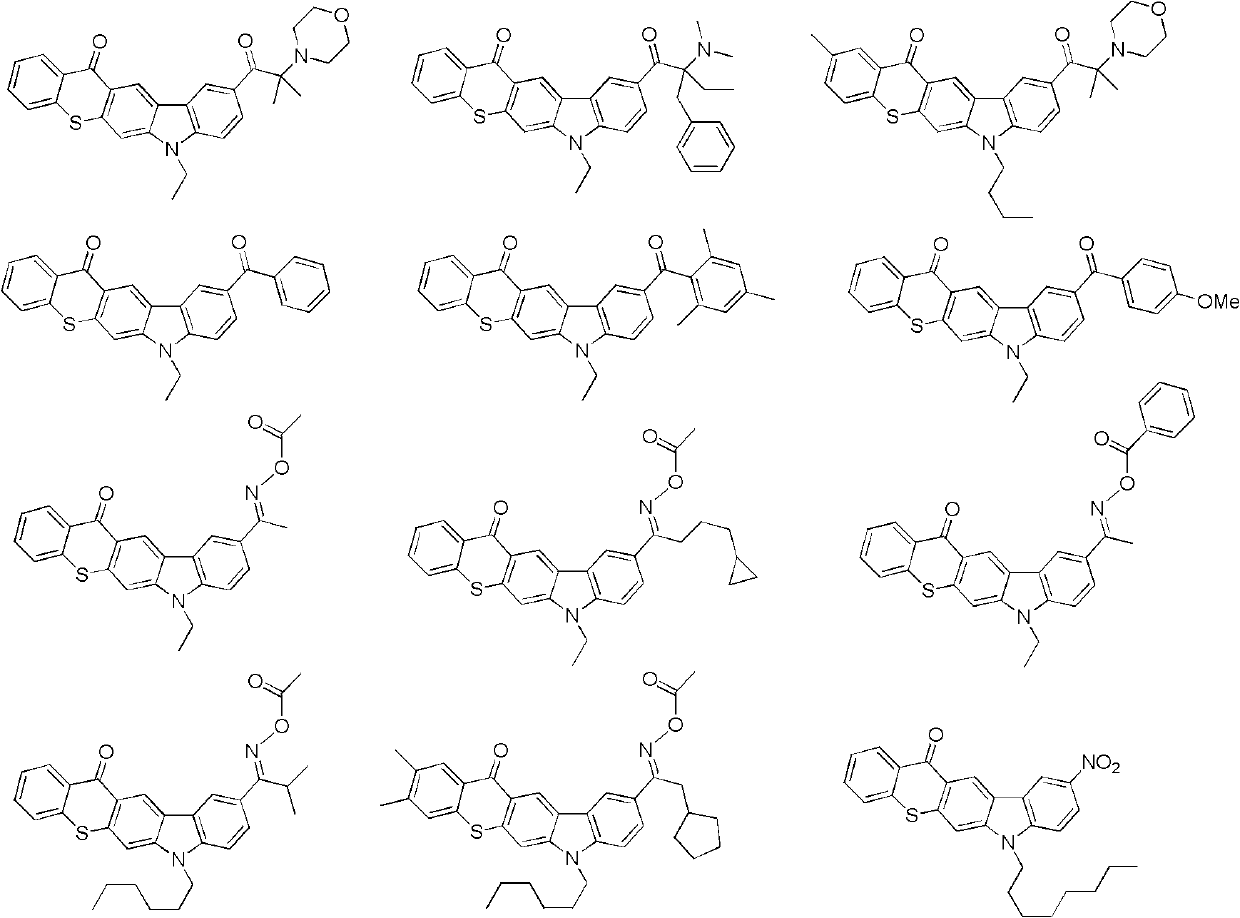
[0031] Example 1: The preparation method of carbazole ring-derived polyfunctional aromatic ketone compound---aminoketone (compound of general formula II).
[0032]
[0033] The first step.1, the synthesis of 6-biscarbazole hexane: under nitrogen protection, get 40.3 grams of carbazole and place in 500 milliliters of dry DMF, add 10.6 grams of NaH (60% paraffin oil dispersion) in small batches powder. After stirring and reacting at room temperature for 2 hours, 29.4 g of 1,6-dibromohexane was added dropwise, and the stirring reaction was continued overnight. The reaction solution was poured into half-saturated brine, extracted 3 times with ethyl acetate, the organic phases were combined, washed 2 times with water, dried over magnesium sulfate, the solvent was spin-dried, and the residue was recrystallized in glacial acetic acid to obtain 42.2 grams of a near-white solid product, 84 % yield.
[0034] The second step. Friedel-Crafts acylation reaction of 1,6-biscarbazole hexan...
Embodiment 2
[0036] Embodiment two: the preparation method of aryl ketone
[0037]
[0038] With reference to the second step conditions of Example 1, under the protection of nitrogen, 13.8 grams of 1,6-biscarbazole hexane and 30.0 grams of 1,3,5-mesoyl chloride were placed in 500 milliliters of dichloroethane. Slowly add 24.2 g of anhydrous aluminum trichloride powder under strong stirring (note that the HCl gas emitted by the reaction is absorbed with lye), and the mixed solution is stirred and reacted at room temperature for 10 hours, and then weakly refluxed for 2 hours. The system was cooled to room temperature, diluted with 700 ml of dichloroethane and poured into a large amount of 2N dilute hydrochloric acid under rapid stirring, the solid was filtered off, the filtrate was layered, the organic phase was washed with saturated brine, dried over anhydrous magnesium sulfate, evaporated After the solvent, the crude product was recrystallized in ethanol to obtain 24.5 g of the aryl ke...
Embodiment 3
[0039] Embodiment three: the preparation method of aminoketone
[0040]
[0041] The first step: under the protection of nitrogen, take 13.6 grams of 1,6-biscarbazole hexane and 13.1 grams of 1,3,5-mesoluoyl chloride in 700 ml of dichloroethane, and slowly add them under vigorous stirring 10.2 grams of anhydrous aluminum trichloride powder (note the HCl gas emitted by the absorption reaction with lye), the mixed solution was stirred and reacted for 6 hours, and then 7.7 grams of isobutyryl chloride and 11.1 grams of anhydrous aluminum trichloride powder were added successively, and the reaction system After stirring overnight, it was diluted with 400 ml of dichloroethane, the mixture was poured into a large amount of 2N dilute hydrochloric acid, the solid was filtered off, the filtrate was layered, the organic phase was washed with saturated brine, dried over anhydrous magnesium sulfate, and the crude product was obtained after distilling off the solvent. Recrystallization ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com