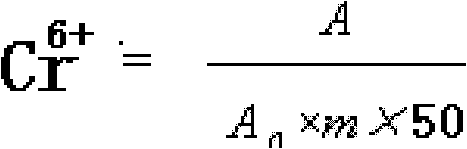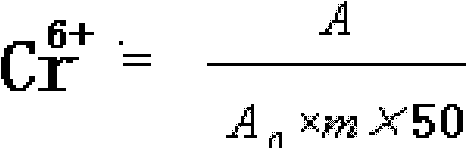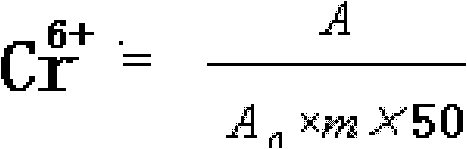Detection method of hexavalent chromium content
A detection method and technology of chromium content, applied in the preparation of test samples, material analysis by observing the influence of chemical indicators, and analysis by making materials undergo chemical reactions, etc., to achieve high accuracy, easy operation, heavy weight, etc. good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Cr in embodiment 1 chromium pyridinecarboxylate raw powder 6+ Determination of
[0021] 1 Reagents and solutions
[0022] 1.1 Sulfuric acid (1+1); 98% concentrated sulfuric acid and water are prepared according to the volume ratio of 1:1
[0023] 1.2 Diphenylcarbazide acetone solution: 5g / L;
[0024] 2.1 Chromium standard stock solution: Weigh 0.1415g of potassium dichromate baked at 105°C to constant weight, dissolve in water, transfer to a 500mL volumetric flask, and dilute to the mark. This solution contains 0.10mg of chromium per ml;
[0025] 2.2 Chromium standard solution: pipette 10ml of chromium stock solution into a 100mL volumetric flask and dilute to the mark with water. This solution contains 10μg of chromium per ml.
[0026] 3. Determination of hexavalent chromium
[0027] 3.1 Weigh 2.0000g (accurate to 0.0001) of the original chromium pyridinecarboxylate powder sample, add it to a 150mL conical flask, add 40mL of water, 20mL of sulfuric acid (1+1), boil...
Embodiment 2
[0037] Cr in embodiment 2 9.9% (chromium content) chromium picolinate and 10% chromium nicotinate 6+ determination
[0038] 1 Weigh 2.000--4.0000g of the sample (accurate to 0.0001), add it to a 150mL Erlenmeyer flask, add 40mL of water, 20mL of sulfuric acid (1+1), boil on an electric stove for 5min, cool naturally, transfer to a 100mL volumetric flask, Make up to volume, shake well and filter.
[0039] Draw 10mL of filtrate, add it to a 50mL volumetric flask, add 0.5mL sulfuric acid (1+1), 2mL diphenylcarbazide acetone solution, and dilute to the mark with water. Take another 50mL volumetric flask, add 0.5mL sulfuric acid (1+1), 2mL diphenylcarbazide acetone solution, and dilute to the mark with water. as blank. Use a blank as a comparison, and immediately measure its absorbance A at a wavelength of 540nm.
[0040] Take 2mL of chromium standard solution (10μg / mL) and put it into a 50mL volumetric flask, add 0.5mL of sulfuric acid (1+1), 2mL of diphenylcarbazide acetone s...
Embodiment 3
[0048] Example 3 Cr in 0.1% (chromium content) chromium picolinate and chromium nicotinate 6+ determination
[0049] 1 Sample 2.000--5.0000g (accurate to 0.0001), put it into a 150mL conical flask, add 40mL of water, 20mL of sulfuric acid (1+1), boil on an electric stove for 5min, cool naturally, transfer to a 100mL volumetric flask, and constant volume , shake well and filter.
[0050] Draw 10mL of filtrate, add it to a 50mL volumetric flask, add 0.5mL sulfuric acid (1+1), 2mL diphenylcarbazide acetone solution, and dilute to the mark with water. Take another 50mL volumetric flask, add 0.5mL sulfuric acid (1+1), 2mL diphenylcarbazide acetone solution, and dilute to the mark with water. as blank. Use a blank as a comparison, and immediately measure its absorbance A at a wavelength of 540nm.
[0051] Take 2mL of chromium standard solution (10μg / mL) and put it into a 50mL volumetric flask, add 0.5mL of sulfuric acid (1+1), 2mL of diphenylcarbazide acetone solution, dilute to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com