Naphthalene derivative metal ion fluorescent probe and its application
A metal ion and fluorescent probe technology, applied in the field of biochemistry, can solve the problems of changing spectral properties, difficult to ensure the accuracy of detection results, etc., and achieve the effects of good selectivity, high sensitivity and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] R in the general formula 2 is pyridyl, R 1 , R 3 Fluorescent probes for phenyl groups A .
[0023] Synthetic procedure: Dissolve 630.8 mg of 1,3-diphenylacetone in 6 mL of ethanol, and then add 636.6 mg of naphthoethylenedione. After all dissolved, 187.7 mg of KOH solid was added, refluxed for 5 min, cooled and placed in the refrigerator for 5 h, filtered with suction, and washed with ethanol to obtain 855.3 mg of black-purple solid with a yield of 73.9%.
[0024] The intermediate product is characterized as follows:
[0025] 1 H-NMR: δ H (400 MHz; CDCl 3 ; ppm) 8.08 (m, 2H), 7.87 (m, 6H), 7.61(m, 2H), 7.55(m, 4H), 7.43 (m, 2H). 13 C-NMR: δ C (100 MHz, CDCl 3 ): 154.21, 132.14, 131.54, 131.44, 129.07, 128.58, 128.42, 128.29, 127.75, 121.66, 120.93.
[0026]Under the protection of argon, 630.6 mg of the above product was dissolved in 6 mL of o-xylene, pumped three times, and 0.20 mL of 2-alkynylpyridine was added, heated to 150 °C, and refluxed for 15 h. Na...
Embodiment 2
[0029] R in the general formula 2 is the anilino group, R 1 , R 3 Fluorescent probes for phenyl groups B
[0030] 2-alkynylpyridine is replaced by 2-alkynylaniline, and the synthesis steps are the same as in Example 1.
[0031] fluorescent probe B Yield 70.2%. 1 H NMR (400 MHz, CDCl 3 , ppm): 3.60(s,2H), 6.50(d,2H), 6.70(s,1H), 7.00(d,2H), 7.27-7.75(m,17H). 13 C NMR (400 MHz, CDCl 3 , ppm): 114.50(2C), 122.70, 123.26, 126.44, 126.52, 127.15, 127.51, 127.59, 127.73, 128.51(2C), 128.60(2C), 129.14(2C), 129.68, 0( ), 131.09, 131.42, 133.11, 135.11, 136.04, 136.13, 136.76, 137.89, 138.23, 139.70, 140.69, 140.93, 144.53. ESI-MS: m / z = 445.1 H]24 [M + .
Embodiment 3
[0032] Embodiment 3 Application of derivative fluorescent probe of the present invention
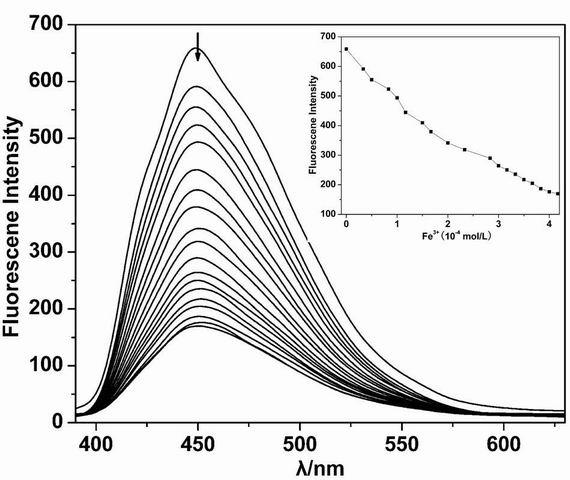
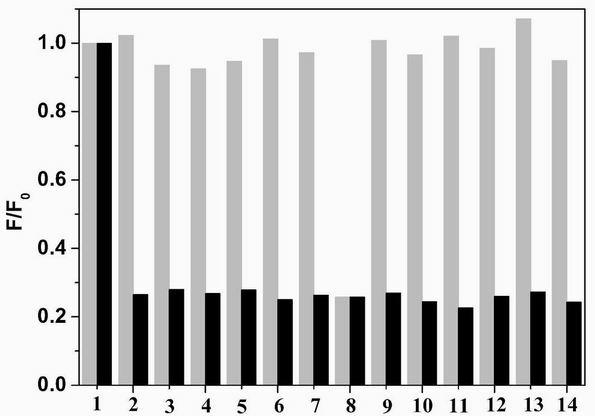
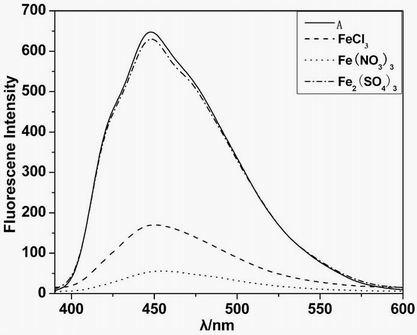
[0033] fluorescent probe A. B. Shows high sensitivity for iron and copper ions, such as figure 1 , 5 shown; the difference is the fluorescent probe A The fluorescence of the iron ion is quenched, while the fluorescent probe B significantly enhances the fluorescence of the iron ion. The above experiments have investigated the effects of complex anions, interfering ions and other factors. Depend on image 3 , 7 It can be seen that anions and interfering ions have no effect on the recognition of iron ions and copper ions by the fluorescent probes A and B of the present invention, and have good selectivity and sensitivity. It will be applied to the identification and detection of metal ions in biological tissues and environmental sewage, and has a good application prospect.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com