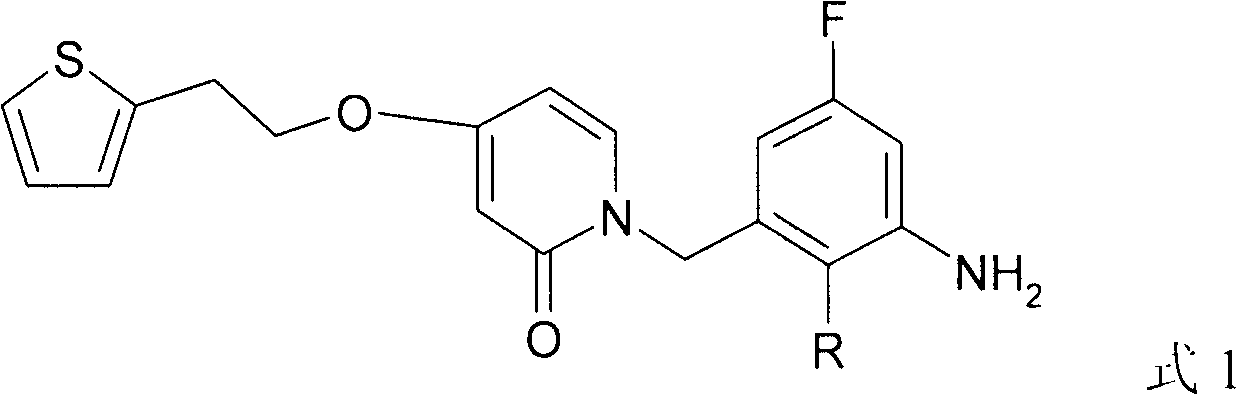
A New Antibacterial Compound Containing Fluorine
A compound and hydrate technology, applied in antibacterial drugs, medical preparations containing active ingredients, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
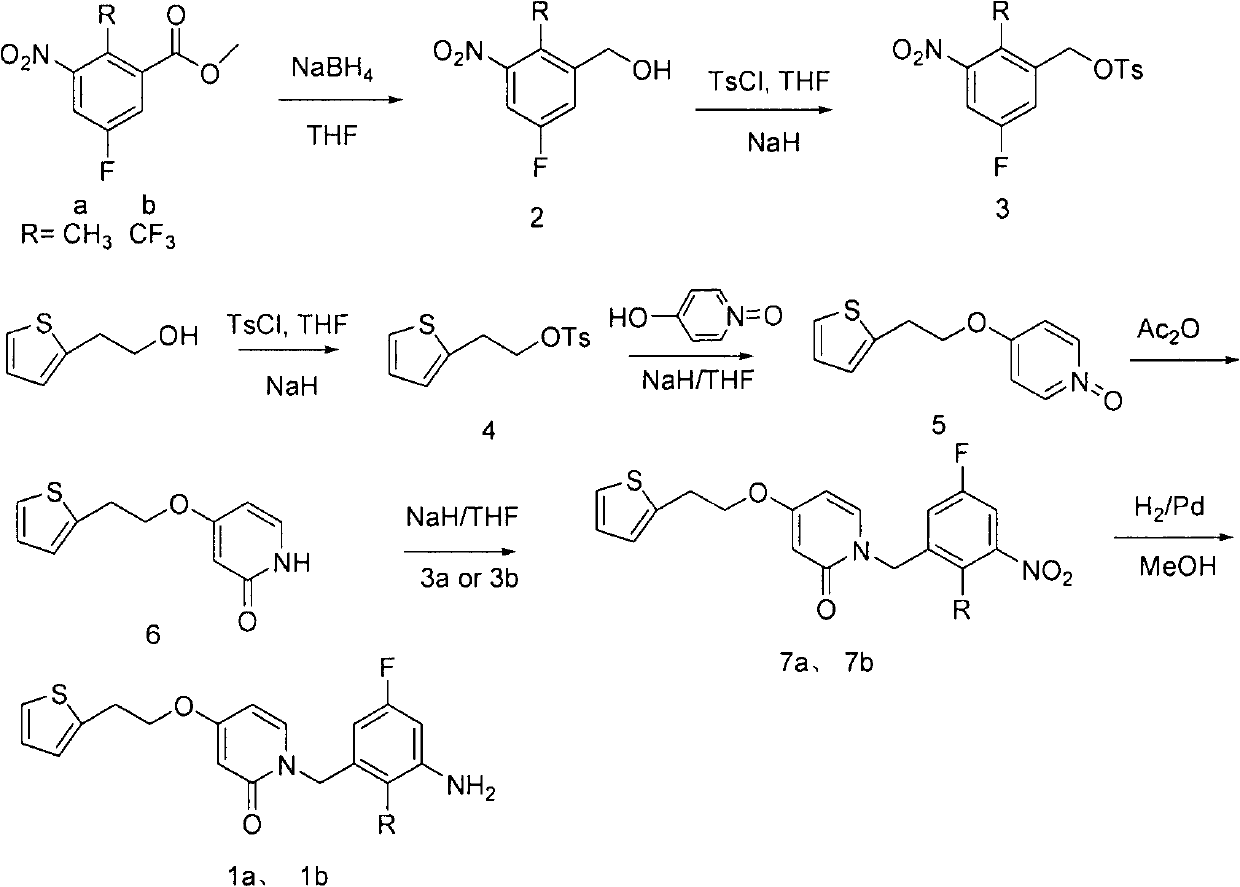
[0010] Embodiment 1: the preparation of formula 1 compound
[0011] 1. Synthesis of Compound 2a, 2b
[0012] Accurately weigh 4.26 grams of raw material a and dissolve in 20.0 mL of THF, add .4 grams of anhydrous Lithium chloride, add 1.0 g of sodium borohydride in batches at 0°C, then move the reaction solution to an oil bath to reflux for 3 hours, stop heating, cool to room temperature, add 20.0 mL of saturated The ammonium solution was separated, the organic layer was separated, the solvent was distilled off under reduced pressure, and 3.5 g of a crude product of compound 2a was obtained after vacuum drying, with a yield of 95%. The crude product was directly used in the next step without purification. MS (m / z): 185.05.
[0013] Using the same method as starting material b, 4.6 g of compound 2b was obtained with a yield of 96%, MS (m / z): 240.12.
[0014] 2. Synthesis of compounds 3a and 3b
[0015] Dissolve the crude product 2a from the previous step in 20.0 mL of THF...
Embodiment 2
[0029] Embodiment 2: the antibacterial activity research of formula 1 compound
[0030] 2.1 Materials and methods
[0031] Antibacterial drug: compound 1a (compound R=-CH of formula 1 3 ) Synthesized by Guangdong Zhongke Pharmaceutical Research Co., Ltd.
[0032] Control drug: desmethylvancomycin hydrochloride was purchased from North China Pharmaceutical Group
[0033] Tested strains: 101 strains of MRSA and 105 strains of MSSA were donated by the School of Pharmacy, Sun Yat-sen University, and determined according to the NCCLS standard after identification.
[0034] Culture medium: purchased from BioMérieux, France, batch number: 811813401.
[0035] Method: adopt double agar dilution method to measure compound 1a (formula 1 compound R=-CH 3 ), and the minimum inhibitory concentration (MIC) of demethylvancomycin hydrochloride to all strains. That is, first dilute the two antibiotics to 12 concentrations with sterile phosphate buffer solution of different concentrations a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com