High-efficiency metal complex catalysts for the polymerization of caprolactone and lactide
A technology of metal complexes and metals, applied in the direction of magnesium organic compounds, calcium organic compounds, zinc organic compounds, etc., can solve the problems of wide product molecular weight distribution, narrow activity, narrow molecular weight distribution, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0018] Correspondingly, the present invention also provides a method for preparing the above two metal complexes, comprising:
[0019] Under anhydrous, oxygen-free and stirring conditions, the ligands and organometallic compounds MX shown in formulas (V), (VIa'), (VIb') and (VIc') 2 (VII) A substitution reaction occurs in an organic solvent to generate a metal complex of formula (I) or formula (II);
[0020]
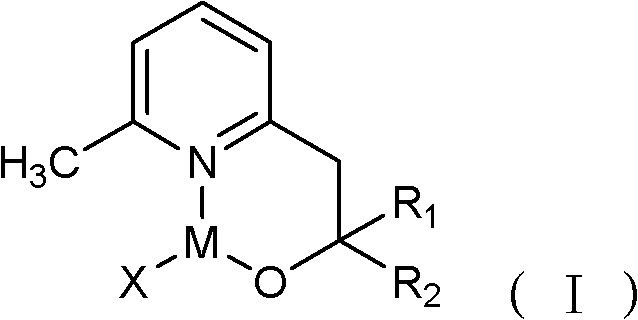
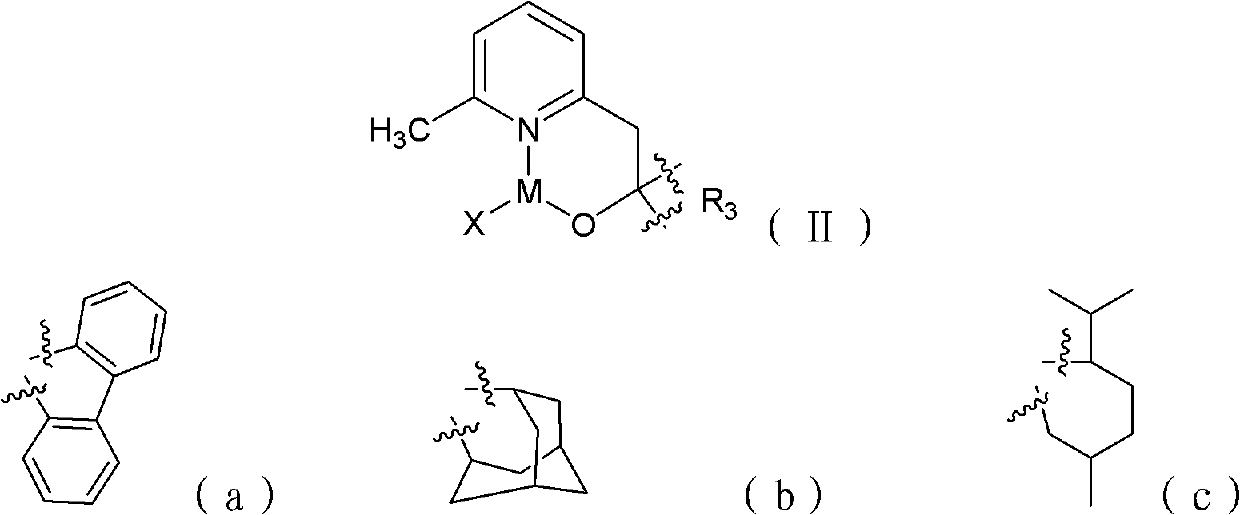
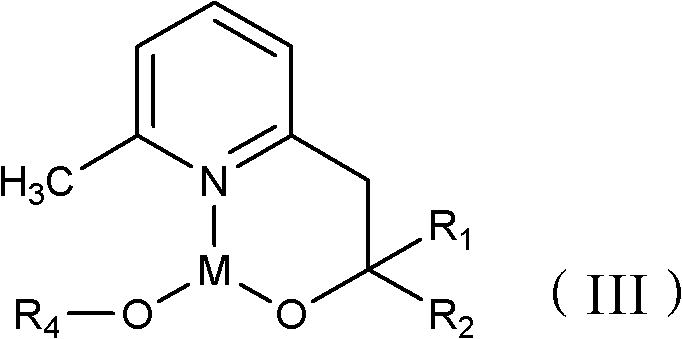
[0021] Among them, R 1 and R 2 Each is independently hydrogen and C1-C30 chain alkane, phenyl or phenyl containing C1-C6 chain alkane group; X is C1-C30 straight-chain or branched-chain alkyl, or aliphatic amino;
[0022] M is the main group metal or transition metal.
[0023] As can be seen from the foregoing scheme, the reaction scheme of the above-mentioned metal complex is as follows:
[0024]
[0025] or
[0026] Since the metal complex is sensitive to air, the reaction should be carried out in an anhydrous, oxygen-free, and rapid stirring condition, an...
Embodiment 1
[0048] Example 1 Preparation of metal complexes A1~A6, B1~B6, F1~F6, J1~J6, K1~K6
[0049] Dissolve 1g of ligand 2-(6-methyl-2-pyridyl)-1-ethanol in 2ml of toluene to obtain a ligand solution; dissolve 7.4ml of dibutylmagnesium solution in 4ml of toluene to obtain dibutyl Magnesium solution in toluene.
[0050] Under anhydrous and oxygen-free conditions, at 25°C, slowly add the above-mentioned ligand solution dropwise to the toluene solution of dibutylmagnesium under rapid stirring and react for 1 hour to end the reaction. The reaction process is as follows:
[0051]
[0052] The reaction mixture was vacuum filtered to obtain a yellow viscous solid, which was washed with 10 ml of hexane solvent to obtain 1.09 g of white solid powder complex A1 with a yield of 69%.
[0053] The product that present embodiment is made carries out elemental analysis, and its molecular formula is C 12 h 19 MgNO: C, 66.24; H, 8.80; Mg, 11.17; N, 6.44; O, 7.35.
[0054] The preparation method...
Embodiment 2
[0059] Example 2 Preparation of metal complexes C1~C6, D1~D6, E1~E6
[0060] Dissolve 1 g of complex A prepared in Example 1 in 2 ml of toluene to obtain a ligand solution; dissolve 2.8 g of isopropanol in 3 ml of toluene to obtain a toluene solution of isopropanol.
[0061] Under anhydrous and oxygen-free conditions, slowly add the above ligand solution dropwise to the toluene solution of isopropanol under rapid stirring at 25°C and react for 1 hour to end the reaction. The reaction process is as follows:
[0062]
[0063] The reaction mixture was vacuum filtered to obtain a white viscous solid, which was washed three times with 10 ml of hexane solvent to obtain 0.91 g of white solid powder complex C1 with a yield of 90%.
[0064] The product that present embodiment is made carries out elemental analysis, and its molecular formula is C 11 h 17 MgNO 2 : C, 60.17; H, 7.80; Mg, 11.07; N, 6.38; O, 14.57.
[0065] The preparation method of complexes C2~C6 is the same as tha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com