A kind of oxaliplatin injection
An oxaliplatin and injection technology, applied in the field of pharmaceutical preparations, can solve the problems of increasing the risk of microbial contamination, sterilization failure, sample precipitation, etc., and achieve the effects of meeting industrial preparation requirements, low manufacturing cost, and simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Accurately weigh 5g of oxaliplatin into the container, then add 900ml of water for injection at 50°C, stir until the oxaliplatin is completely dissolved, and cool to room temperature; add 10ml of acetic acid-sodium acetate buffer solution, wherein: acetic acid in the buffer solution The molar ratio to sodium acetate is 4:1, and the molar concentrations of acetic acid and sodium acetate are 0.2M and 0.05M respectively; then make up to 1000ml with water for injection; filter, fill, stopper, cover, and finally extinguish with damp heat at 121°C 15 minutes to obtain the oxaliplatin injection of the present invention, which is referred to as preparation 1.
Embodiment 2
[0042] Preparation 4, preparation 5 and preparation 6 were prepared according to the preparation process described in Example 1, and the specific formulations are shown in Table 3.
[0043] table 3
[0044]
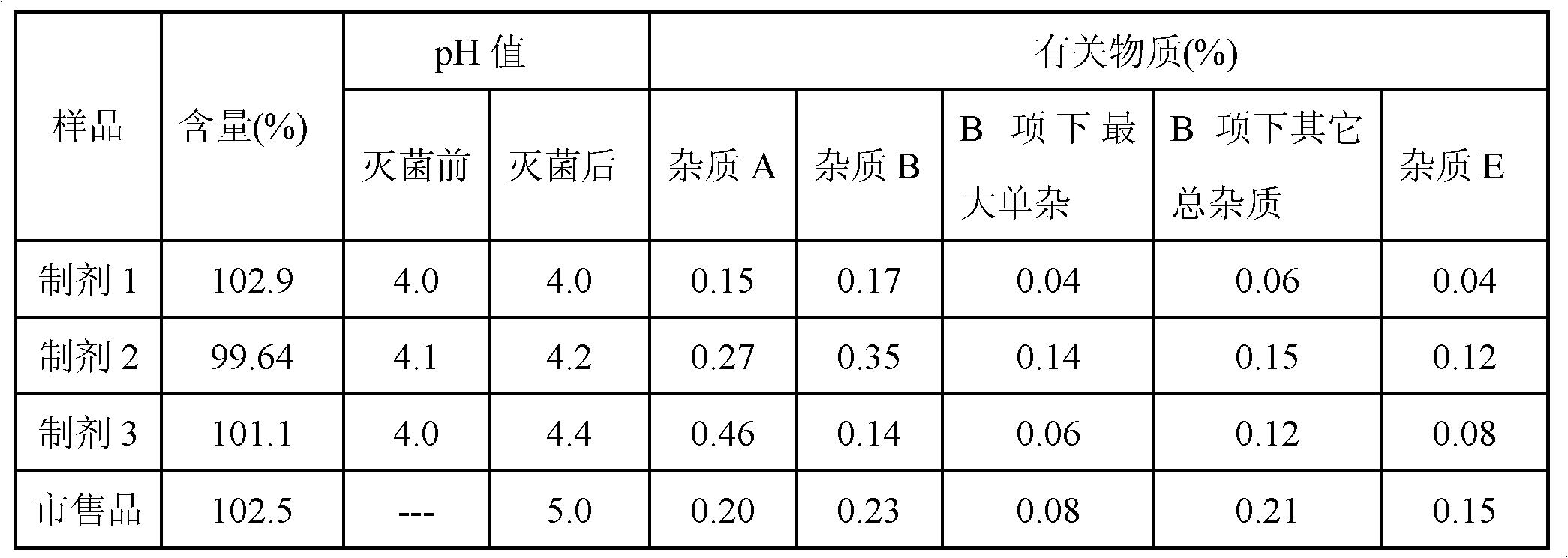
[0045] The test results of the content, pH value and the amount of related substances of the above three preparations are shown in Table 4.
[0046] Table 4
[0047]
[0048] It can be seen from Table 4 that when acetic acid or sodium acetate is used alone as a stabilizer, the pH value changes before and after sterilization, and the related substances after sterilization are relatively large; especially when acetic acid is used alone as a stabilizer, the relevant substances after sterilization The growth of substances is particularly obvious; and the oxaliplatin injection prepared by using the buffer solution of acetic acid-sodium acetate as a stabilizer in the present invention has stable and superior quality before and after sterilization.
[0049] Accelerated s...
Embodiment 3
[0054] Preparation 7, preparation 8, preparation 9, preparation 10 and preparation 11 were prepared according to the preparation process described in Example 1, and the specific formulations are shown in Table 6.
[0055] Table 6
[0056]
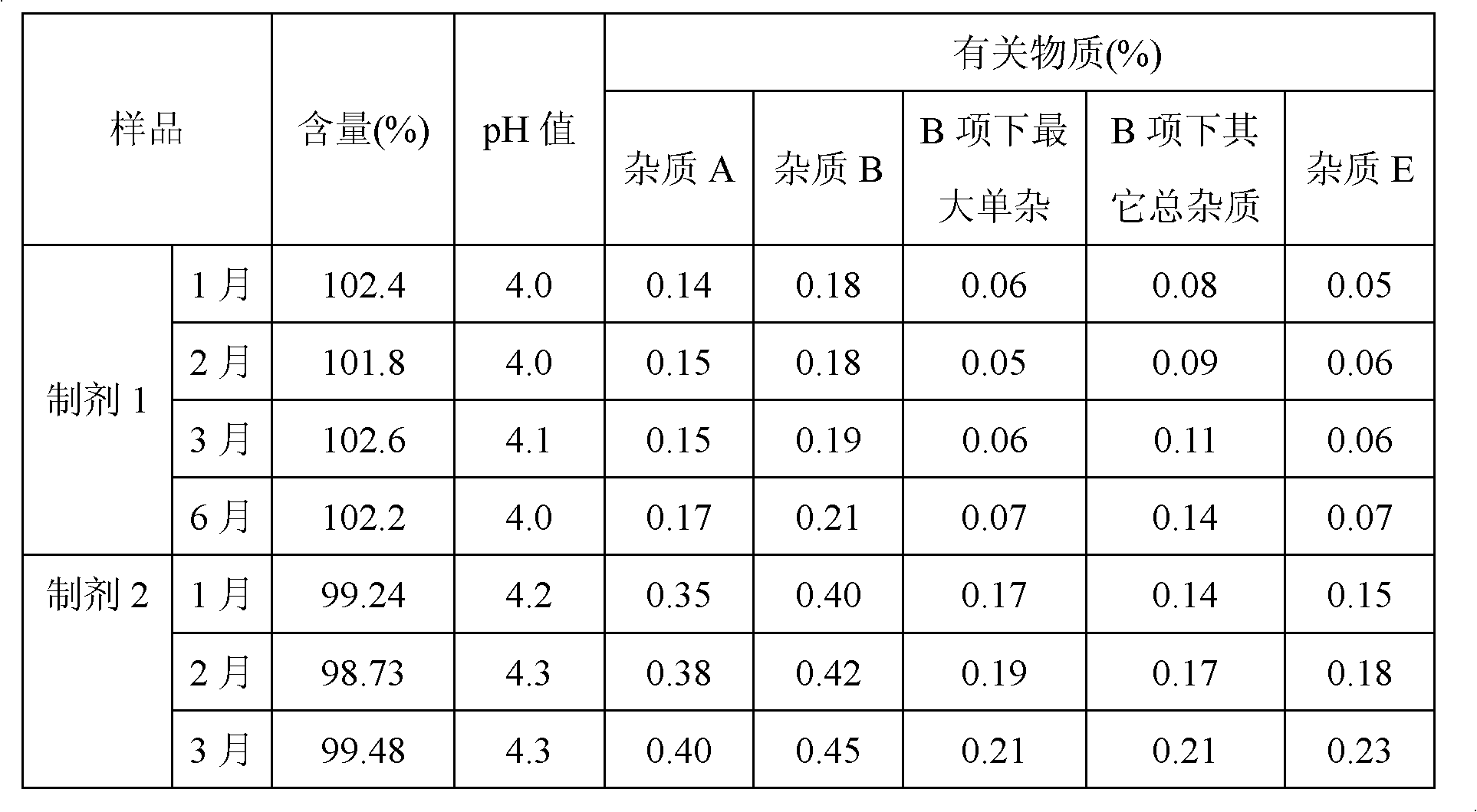
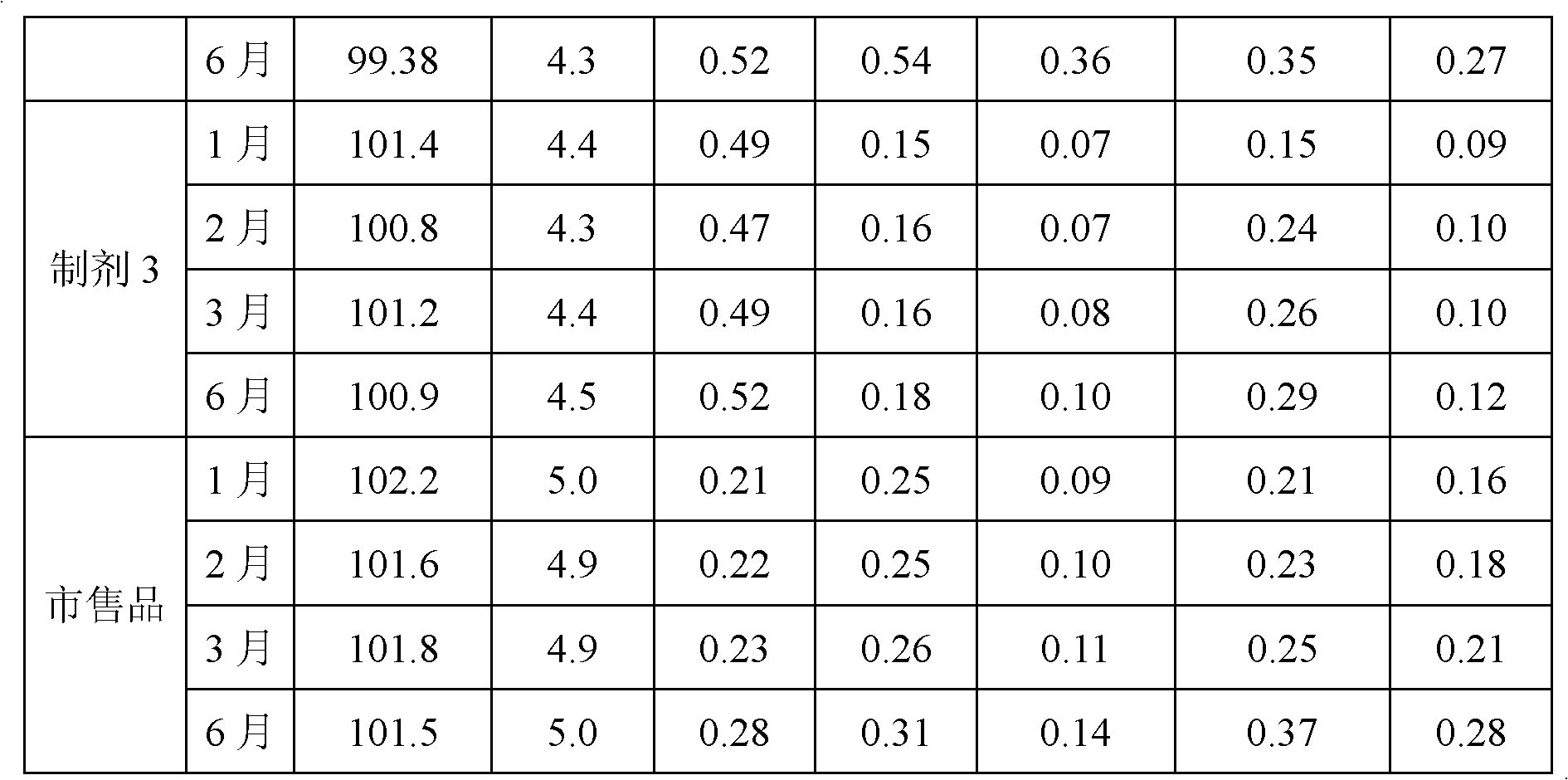
[0057] The test results of the content, pH value and the amount of related substances of the above five preparations are shown in Table 7.
[0058] Table 7
[0059]
[0060] It can be seen from Table 7 that the oxaliplatin injections prepared from the buffer solution formed with the molar ratio of acetic acid and sodium acetate as 1:1 to 5:1 all have stable quality; especially when the molar ratio of acetic acid and sodium acetate is 3 : 1~5: 1 The oxaliplatin injection prepared by the buffer solution that forms has better quality, the oxaliplatin injection that is 3: 1~4: 1 to form the buffer solution that the mol ratio of acetic acid and sodium acetate prepares has best quality.
[0061] Accelerated stability tests were carried o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com