Medicinal composition for high-androgen polycystic ovary syndrome
A high androgen, polycystic ovary technology, applied in drug combinations, medical raw materials derived from arthropods, pharmaceutical formulations, etc., can solve problems such as fetal harm, pelvic adhesions, and ovarian damage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The preparation of embodiment 1 decoction
[0050] Take 0.6g of hippocampus, 15g of fairy spleen, 12g of fritillaria, 15g of psoralen, 12g of tortoise shell, 12g of antler slices, 15g of saponin thorn and 15g of calamus, rinse with water, soak in the container for more than 2 hours, and then fry And the following traditional decocting process, decocting at normal pressure for 20 minutes to filter out the medicinal liquid.
Embodiment 2
[0051] The preparation of embodiment 2 ointment
[0052] Take 6g of hippocampus, 150g of fairy spleen, 120g of fritillaria, 150g of psoralen, 120g of tortoise shell, 120g of antler slices, 150g of saponin thorn and 150g of calamus, rinse with water, soak in the container for more than 2 hours, and then fry and mix The following traditional decoction process, after decocting under normal pressure, filter out the liquid medicine and concentrate the liquid medicine under reduced pressure. During the concentration process of the liquid medicine, add rock sugar, maltose and rice wine and other required sugars and necessary auxiliary materials, and stir to collect the paste .
Embodiment 3
[0053] Embodiment 3 clinical observation
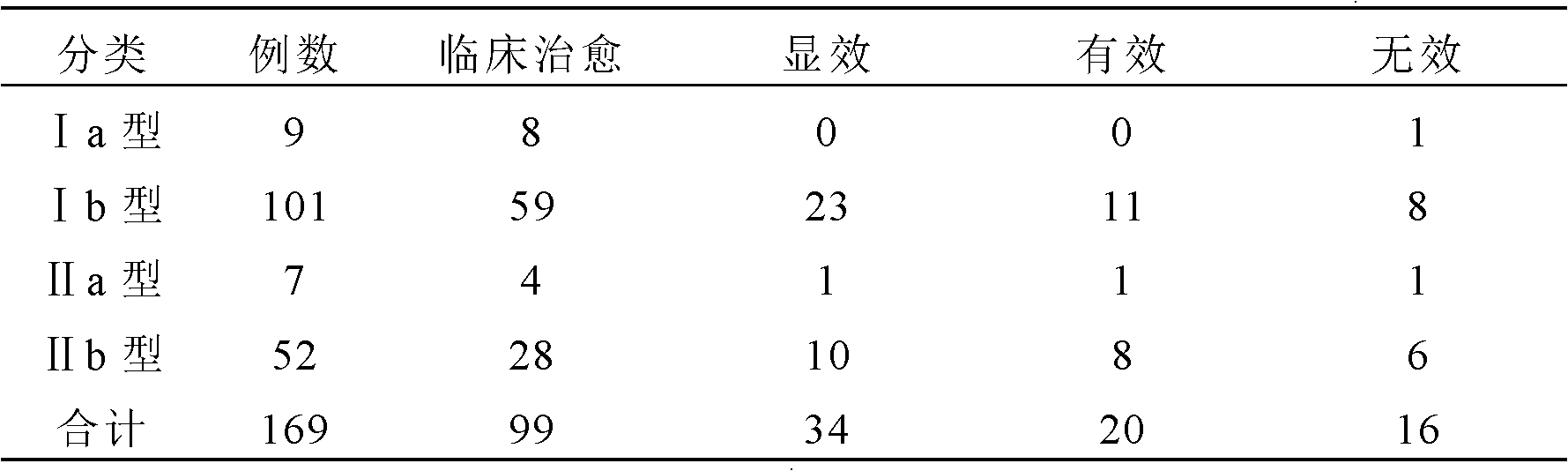
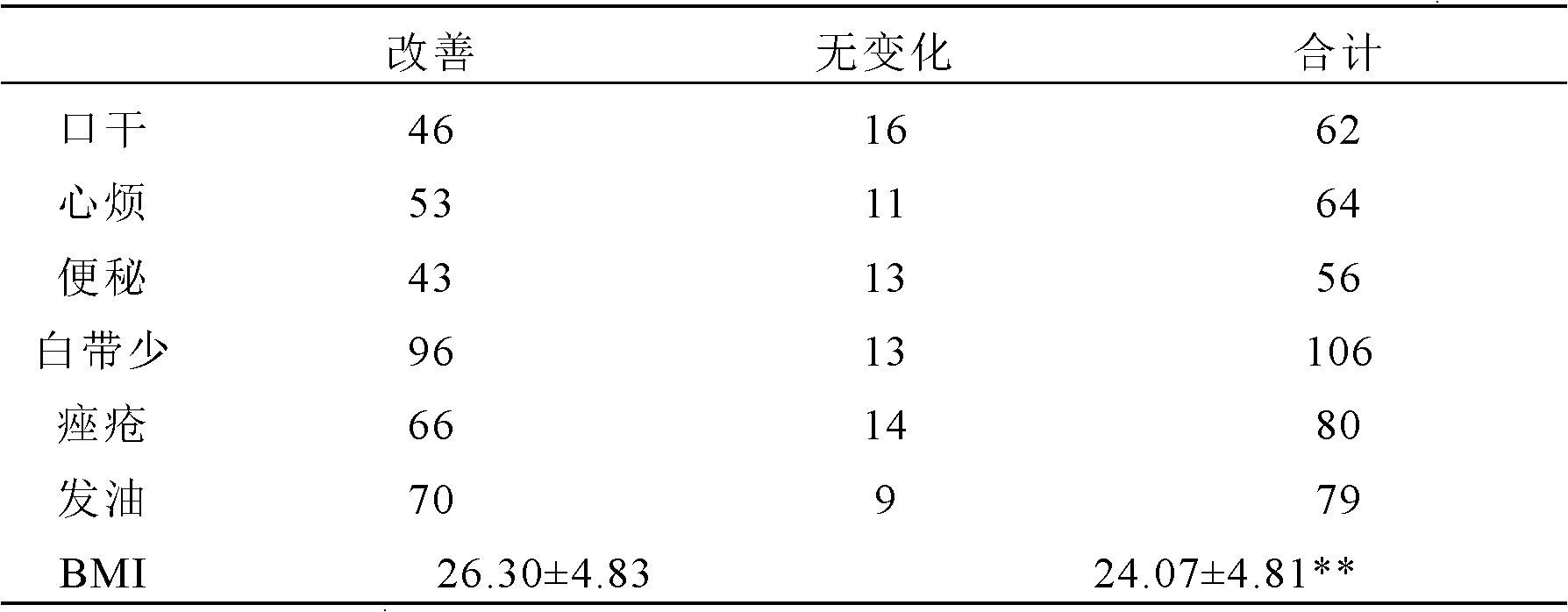
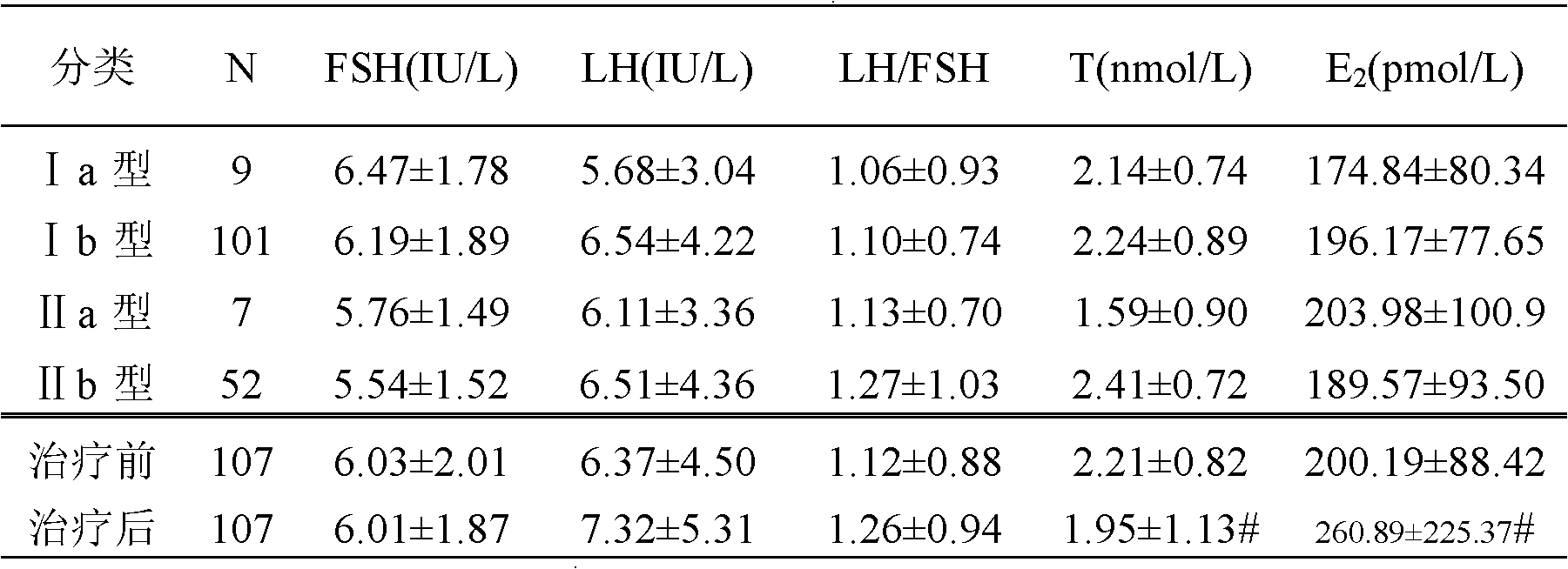
[0054] Patient entry criteria: 1) Basal body temperature (BBT) continued monophasic 2 years after menarche, oligomenorrhea or amenorrhea; 2) Blood androgen increased: blood testosterone (T) increased or testosterone / estradiol logarithmic ratio (logT / E 2 )>0.97; can be combined with increased 17α-hydroxyprogesterone (17α-OHP) and androstenedione; 3) B-ultrasound examination shows that the ovarian volume increases (≥6ml), and the number of follicles with a diameter of 2-9mm on a plane in the cortex> 10; 4) No response to clomiphene treatment, and the hormone therapy used has been stopped for more than 3 months; 5) Congenital adrenal hyperplasia, small follicle syndrome, Cushing's syndrome, androgen-secreting ovarian and adrenal tumors, etc. were excluded disease; 6) aged 18 to 40 years old. All patients have been treated by traditional Chinese medicine and Western medicine for more than half a year, but no ovulation or pregnancy occu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com