Pharmaceutical composition for treating polycystic ovary syndrome (PCOS)
A polycystic ovary and composition technology, applied in the field of polycystic ovary syndrome treatment, can solve problems such as ovarian damage, pelvic adhesions, and fetal harm
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] The preparation of embodiment 1 decoction
[0054] Take 15g Anemarrhena, 12 Fritillaria, 24g Zhishouwu, 12g Ophiopogon japonicus, 12g Tortoise shell, 0.6g hippocampus, 9g Angelica sinensis, 15g Saponaria thorn, and 15g Shichangpu, rinse with water, soak in the container for more than 2 hours, and then use The traditional decocting process of decocting first and then decocting, decocting at normal pressure for 20 minutes to filter out the medicinal liquid.
Embodiment 2
[0055] The preparation of embodiment 2 ointment
[0056] Take 150g Anemarrhena, 120g Fritillaria, 260g Zhishouwu, 120g Ophiopogon japonicus, 120g Tortoise shell, 6g hippocampus, 90g Angelica sinensis, 150g Saponaria thorn, and 150g Shichangpu, rinse with water, soak in the container for more than 2 hours, and then use the first The traditional decoction process of decocting and decocting. After decocting under normal pressure, the liquid medicine is filtered out and the liquid medicine is concentrated under reduced pressure. During the process of concentrating the liquid medicine, sugars and necessary auxiliary materials such as rock sugar, maltose and rice wine are added, and stirred Receive ointment.
Embodiment 3
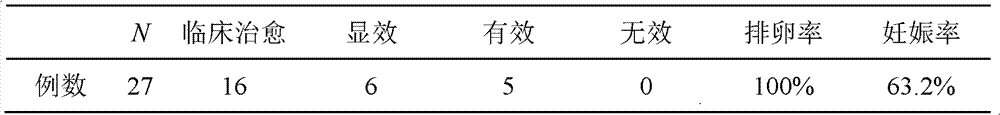
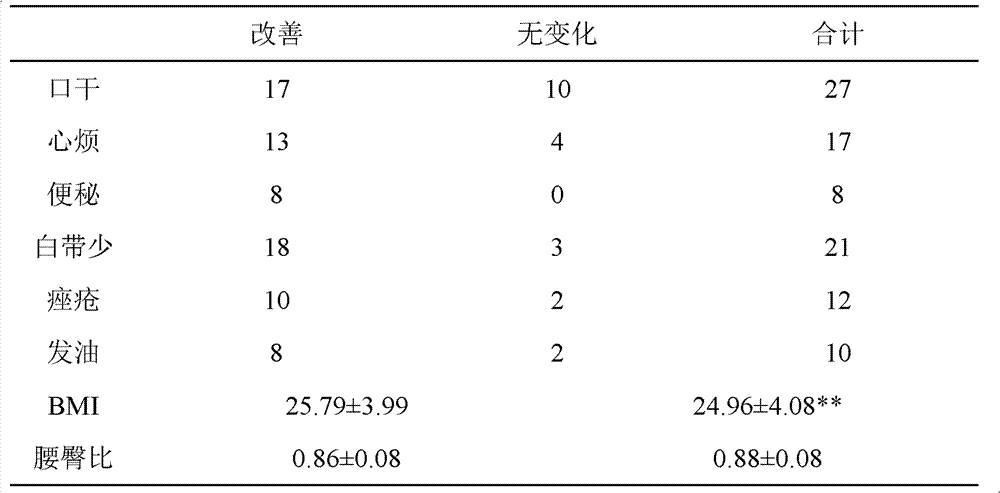
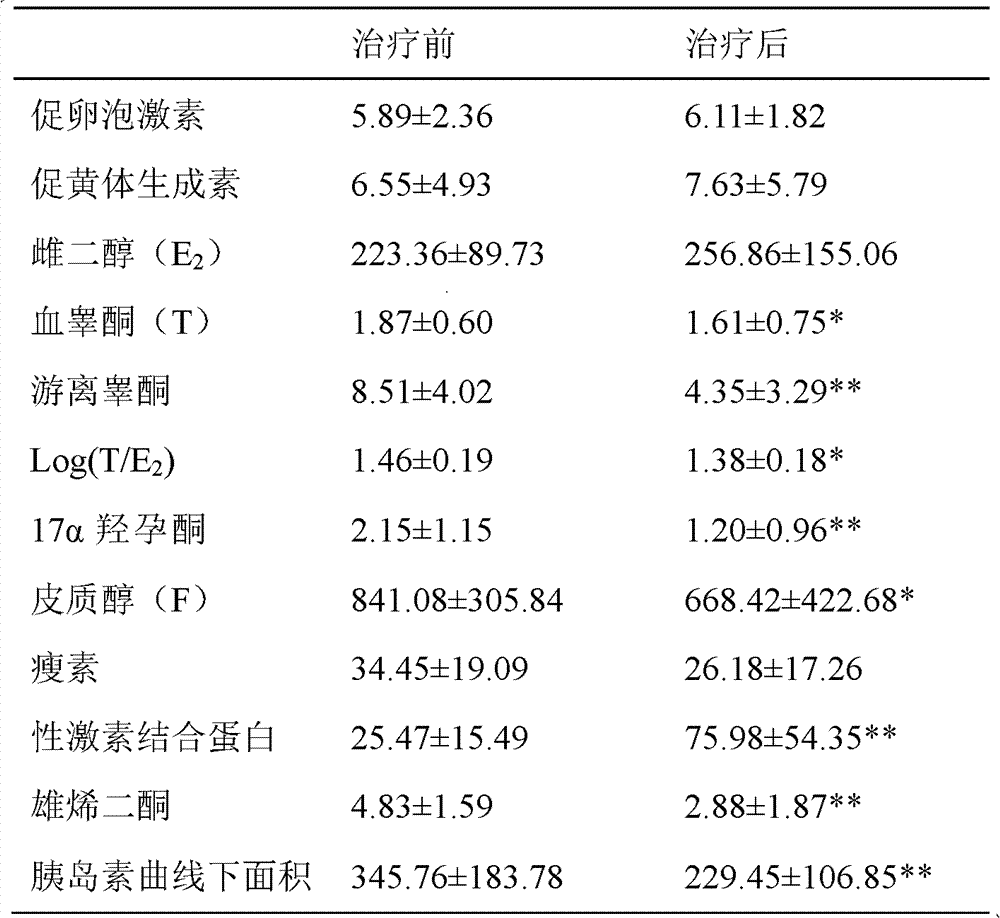
[0057] Embodiment 3 clinical observation
[0058] Patients with kidney yin deficiency and phlegm and blood stasis are included according to the following criteria:
[0059] A) Diagnostic criteria of Western medicine: 1) Menstrual cramps for more than 2 years are still anovulatory menstruation (BBT monophasic), oligomenorrhea or amenorrhea; 2) Blood androgen rise: on the third day of uterine bleeding, blood testosterone (T) rises High or testosterone / estradiol logarithmic ratio (Log(T / E 2 ))>0.97; 17α-hydroxyprogesterone (17α-OHP) and androstenedione can be combined to increase; 3) B-ultrasound examination found that the ovarian volume increased (≥6ml), and the number of follicles with a diameter of 2-9mm on a plane in the cortex >10.
[0060] B) TCM syndrome classification standard: In addition to less menstrual period or amenorrhea, infertility, accompanied by dry mouth, upset, internal heat, leukorrhea, constipation, etc., dark red tongue, less coating, thready pulse.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com