Absorption and Catalytic Decomposition for Ammonia Removal at Low Temperature
A catalytic material, zinc oxide technology, applied in the field of catalysis and environmental science, can solve the problems of limited application and low degradation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
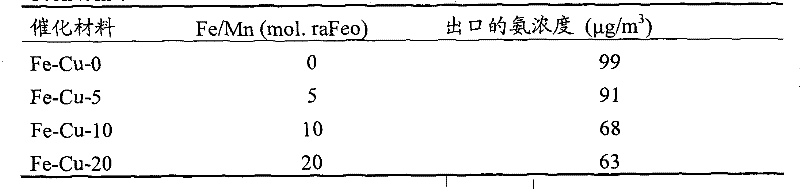
[0022] Copper-iron composite oxide powders are synthesized by soluble copper salts and potassium persulfate (K 2 S 2 o 8 ) mixed with copper sulfate in nitric acid solution, while adding ferric nitrate (Fe(NO 3 ) 2 ), so that the Fe / Cu molar ratio is 0 to 0.20, and the resulting black precipitate is vigorously stirred and refluxed at 100°C for 24h, filtered, washed, dried at 110°C for 12h, and then roasted at 500°C for 6h to obtain a copper-iron composite of iron Oxide powder, respectively marked as Fe-Cu-0, Fe-Cu-5, Fe-Cu-10 and Fe-Cu-20, where Fe-Cu means copper-iron composite oxide, and the number means Fe / Cu total The molar ratio percentage.
[0023] XRD analysis and HRTEM analysis show that the material is copper-iron composite oxide.
[0024] The performance tests of the catalytic materials were carried out in a continuous flow fixed bed reactor. The powdered catalytic material is tableted and crushed to form a 0.25-0.50mm particle sample, and then 0.5g of the part...
Embodiment 2
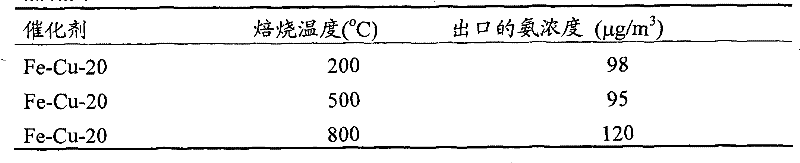
[0026] The synthesis of copper-iron composite oxide powder is with embodiment one, namely by copper salt and potassium persulfate (K 2 S 2 o 8 ) mixed with copper sulfate in nitric acid solution, while adding ferric nitrate (Fe(NO 3 ) 2 ), so that the Fe / Mn molar ratio is 0 to 0.20, the resulting black precipitate is vigorously stirred and refluxed at 100°C for 24h, filtered, washed, dried at 110°C for 12h, and then roasted at 200-800°C for 6h to obtain iron-copper- Iron composite oxide powder, respectively recorded as Fe-Cu-0, Fe-Cu-5, Fe-Cu-10 and Fe-Cu-20, where Fe-Cu represents iron-copper-iron composite oxide, and the number represents Fe / Cu total The molar ratio percentage.
[0027] XRD analysis and HRTEM analysis show that the material is copper-iron composite oxide.
[0028] The performance tests of the catalytic materials were carried out in a continuous flow fixed bed reactor. The powdered catalytic material is tableted and crushed to form a 0.25-0.50mm parti...
Embodiment 3
[0030] 1) Synthesis of copper-iron composite oxide powder
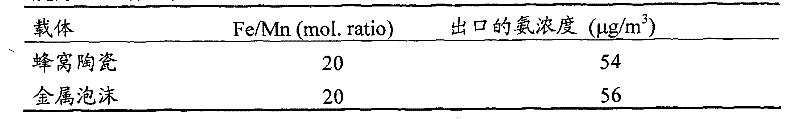
[0031] The synthesis of copper-iron composite oxide powder is the same as example one, that is, the synthesis of iron-copper-iron composite oxide powder is through copper salt and potassium persulfate (K 2 S 2 o 8 ) mixed with copper sulfate in nitric acid solution, while adding ferric nitrate (Fe(NO 3 ) 2 ), so that the Fe / Cu molar ratio is 0 to 0.20, and the resulting black precipitate is vigorously stirred and refluxed at 100°C for 24h, filtered, washed, dried at 110°C for 12h, and then roasted at 500°C for 6h to obtain a copper-iron composite of iron oxide powder.
[0032] 2) Preparation of catalytic materials supported by copper-iron composite oxide honeycomb ceramics.
[0033] Weigh a certain amount of copper-iron composite oxide, add a certain proportion of deionized water and the inorganic binder in claim 9, and stir at a high speed for 1-24 hours to obtain a copper-iron composite oxide slurry with a cert...
PUM
| Property | Measurement | Unit |
|---|---|---|
| clearance rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com