Technique for catalytic removal of ozone at room or low temperature
A catalytic material, manganese oxide technology, applied in the field of catalysis and environmental science, to achieve high processing efficiency, complete processing, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
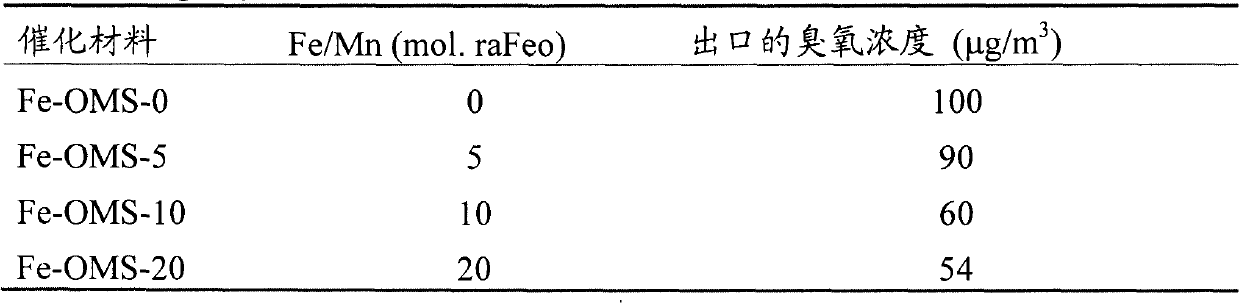
[0021] Iron-doped manganese oxide molecular sieve powders were synthesized by potassium permanganate (KMnO 4 ) and potassium persulfate (K 2 S 2 o 8 ) in nitric acid solution to oxidize manganese sulfate (MnSO 4 ·H 2 O), while adding iron nitrate (Fe(NO 3 ) 2 ), so that the Fe / Mn molar ratio is 0-0.20, the resulting black precipitate is vigorously stirred and refluxed at 100°C for 24h, filtered, washed, dried at 110°C for 12h, and then roasted at 500°C for 6h to obtain iron-doped manganese oxide Molecular sieve powder, respectively marked as Fe-OMS-0, Fe-OMS-5, Fe-OMS-10 and Fe-OMS-20, where Fe-OMS means iron-doped manganese oxide molecular sieve, and the number means Fe / Mn total The molar ratio percentage.
[0022] XRD analysis and HRTEM analysis indicated that the material was Hollandite type manganese oxide.
[0023] The performance tests of the catalytic materials were carried out in a continuous flow fixed bed reactor. The powdered catalytic material is tableted ...
Embodiment 2
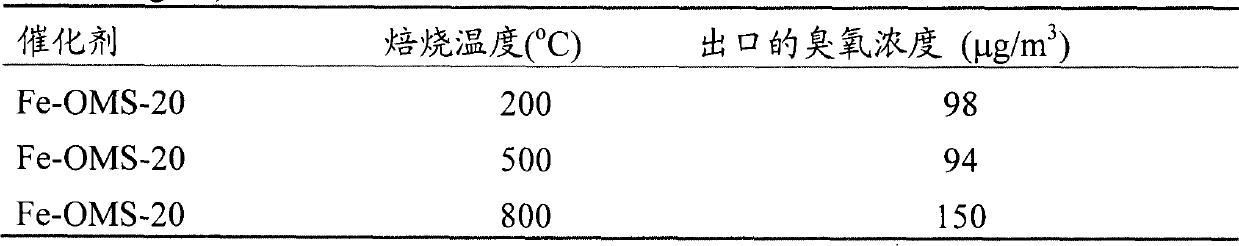
[0025] The synthesis of the iron-doped manganese oxide molecular sieve powder is the same as in Example one, namely by potassium permanganate (KMnO 4 ) and potassium persulfate (K 2 S 2 o 8 ) in nitric acid solution to oxidize manganese sulfate (MnSO 4 ·H 2 O), while adding iron nitrate (Fe(NO 3 ) 2 ), so that the Fe / Mn molar ratio is 0 to 0.20, and the resulting black precipitate is vigorously stirred and refluxed at 100°C for 24h, filtered, washed, dried at 110°C for 12h, and then roasted at 200-800°C for 6h to obtain iron-doped Manganese oxide molecular sieve powder, respectively recorded as Fe-OMS-0, Fe-OMS-5, Fe-OMS-10 and Fe-OMS-20, where Fe-OMS means iron-doped manganese oxide molecular sieve, and the number means Fe / Mn total The molar ratio percentage.
[0026] XRD analysis and HRTEM analysis indicated that the material was Hollandite type manganese oxide.
[0027] The performance tests of the catalytic materials were carried out in a continuous flow fixed bed ...
Embodiment 3
[0029] 1) Synthesis of doped manganese oxide molecular sieve powder
[0030] The synthesis of the manganese oxide molecular sieve powder of doping is the same as example one, that is, the synthesis of the iron-doped manganese oxide molecular sieve powder is through potassium permanganate (KMnO 4 ) and potassium persulfate (K 2 S 2 o 8 ) in nitric acid solution to oxidize manganese sulfate (MnSO 4 ·H 2 O), while adding iron nitrate (Fe(NO 3 ) 2 ), so that the Fe / Mn molar ratio is 0-0.20, the resulting black precipitate is vigorously stirred and refluxed at 100°C for 24h, filtered, washed, dried at 110°C for 12h, and then roasted at 500°C for 6h to obtain iron-doped manganese oxide Molecular sieve powder.
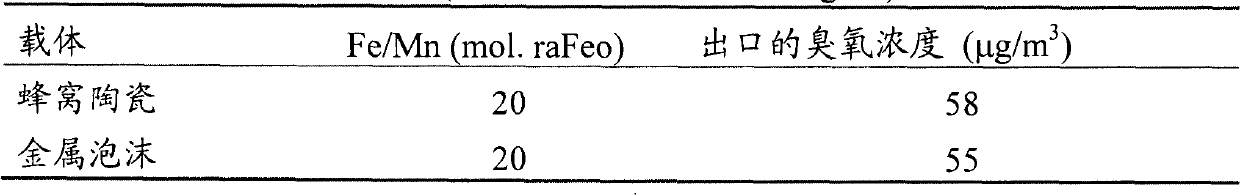
[0031] 2) Preparation of doped manganese oxide molecular sieve honeycomb ceramic monolithic catalytic material.
[0032] Weigh a certain amount of doped manganese oxide molecular sieve, add a certain proportion of deionized water and the inorganic binder in claim 9, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com