Solid Phase Synthesis of Thymosin α1
A technology of solid-phase synthesis and thymosin, which is applied in the field of solid-phase synthesis of thymosin α1, can solve the problems of low condensation efficiency, large influence of spatial structure, unfavorable industrial production, etc., so as to reduce the influence, improve the reaction yield, Less side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: AA (1-27) -Asn(Trt) 28 -Synthesis of CTC resin
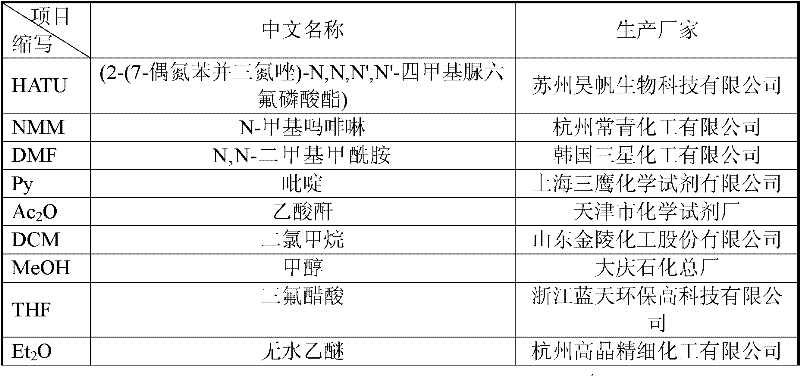
[0031]Weigh 66.7g (40mmol) of Fmoc-Asn(Trt)-CTC resin with a substitution degree of 0.60mmol / g, add it to the reactor, add 500ml DMF, turn on the nitrogen gas and mix the liquid and resin gently from bottom to top, stir and swell Stop after 30 minutes, and remove the liquid by suction filtration. Then add 500ml of 20% piperidine / DMF solution (capping solution) into the reactor, stir and react for 5 minutes under nitrogen protection, remove the liquid, and wash twice with 500ml DMF. Then add 500ml of decapping solution, stir and react for 10 minutes, then remove, wash with 500ml of DMF 8 times, and wash off the Fmoc protecting group. Weigh 68g of Fmoc-Glu(OtBu)-OH, 60.8g of HATU, dissolve in 500ml of DMF, add 26.5ml of cooled NMM under ice bath, activate for 10 minutes, add to the reactor, and react at 15℃~25℃ 2.5-3 hours, subject to ninhydrin colorimetric detection. Then the reaction liquid was removed by ...
Embodiment 2
[0032] Example 2: Ac-AA (1-27) -Asn 28 Preparation of (Trt)-CTC resin
[0033] Prepare the acetylation solution 76ml of acetic anhydride, 65ml of pyridine, and 1000ml of DMF, mix well and add to the reactor, react for 24 hours under the protection of nitrogen, and then remove the acetylation solution by suction filtration. Add 1000ml DMF to wash 6 times, 1500ml DCM to wash 3 times, 1000ml MeOH to wash 3 times, each time for 5 minutes. The resin was removed and dried under reduced pressure to obtain 242.2 g of thymosin α1 peptide resin.
Embodiment 3
[0034] Example 3: Preparation of Crude Peptides by Cleavage of Resin Peptides
[0035] (1) Prepare 2400 ml of lysis solution of trifluoroacetic acid / dimercaptoethanol / water=90:5:5 (v / v), and pre-freeze at -10°C for 12 hours.
[0036] (2) Put 242.2g of peptide resin in a round-bottomed flask, cool in an ice bath, add 2400ml of pre-frozen lysate, remove the ice bath after the addition, and stir at room temperature for 2.5 hours. Then the resin was removed by filtration, and the filtrate was added to 10 times the volume of glacial ether. Settled for 5 hours. Centrifuge and wash 8 times at 4000 rpm. The precipitate was removed and dried under reduced pressure to obtain 73.5 g of crude thymosin α1 peptide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com